References
1. Kim WB, Kim TY, Kwon HS, Moon WJ, Lee JB, Choi YS, et al. Management guidelines for patients with thyroid nodules and thyroid cancer. J Korean Endocr Soc. 2007; 22(3):157–87.

2. Yi KH, Park YJ, Koong SS, Kim JH, Na DG, Ryu JS, et al. Revised Korean Thyroid Association Management Guidelines for patients with thyroid nodules and thyroid cancer. J Korean Thyroid Assoc. 2010; 3(2):65–96.

3. Ito Y, Miyauchi A, Kihara M, Higashiyama T, Kobayashi K, Miya A. Patient age is significantly related to the progression of papillary microcarcinoma of the thyroid under observation. Thyroid. 2014; 24(1):27–34.

4. Sugitani I, Toda K, Yamada K, Yamamoto N, Ikenaga M, Fujimoto Y. Three distinctly different kinds of papillary thyroid microcarcinoma should be recognized: our treatment strategies and outcomes. World J Surg. 2010; 34(6):1222–31.

5. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015; 136(5):E359–86.

6. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016; 26(1):1–133.

7. Yi KH, Kim SY, Kim DH, Kim SW, Na DG, Lee YJ, et al. The Korean guideline for thyroid cancer screening. J Korean Med Assoc. 2015; 58(4):302–12.

8. Gharib H, Papini E, Garber JR, Duick DS, Harrell RM, Hegedus L, et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi Medical Guidelines for clinical practice for the diagnosis and management of thyroid nodules–2016 update. Endocr Pract. 2016; 22(5):622–39.
9. Marqusee E, Benson CB, Frates MC, Doubilet PM, Larsen PR, Cibas ES, et al. Usefulness of ultrasonography in the management of nodular thyroid disease. Ann Intern Med. 2000; 133(9):696–700.

10. Hagag P, Strauss S, Weiss M. Role of ultrasound-guided fine-needle aspiration biopsy in evaluation of nonpalpable thyroid nodules. Thyroid. 1998; 8(11):989–95.

11. Capezzone M, Marchisotta S, Cantara S, Busonero G, Brilli L, Pazaitou-Panayiotou K, et al. Familial non-medullary thyroid carcinoma displays the features of clinical anticipation suggestive of a distinct biological entity. Endocr Relat Cancer. 2008; 15(4):1075–81.

12. Park YJ, Ahn HY, Choi HS, Kim KW, Park DJ, Cho BY. The longterm outcomes of the second generation of familial nonmedullary thyroid carcinoma are more aggressive than sporadic cases. Thyroid. 2012; 22(4):356–62.

13. Mazeh H, Benavidez J, Poehls JL, Youngwirth L, Chen H, Sippel RS. In patients with thyroid cancer of follicular cell origin, a family history of nonmedullary thyroid cancer in one first-degree relative is associated with more aggressive disease. Thyroid. 2012; 22(1):3–8.

14. Robenshtok E, Tzvetov G, Grozinsky-Glasberg S, Shraga-Slutzky I, Weinstein R, Lazar L, et al. Clinical characteristics and outcome of familial nonmedullary thyroid cancer: a retrospective controlled study. Thyroid. 2011; 21(1):43–8.

15. Richards ML. Familial syndromes associated with thyroid cancer in the era of personalized medicine. Thyroid. 2010; 20(7):707–13.

16. Curtis RE, Rowlings PA, Deeg HJ, Shriner DA, Socie G, Travis LB, et al. Solid cancers after bone marrow transplantation. N Engl J Med. 1997; 336(13):897–904.

17. Pacini F, Vorontsova T, Demidchik EP, Molinaro E, Agate L, Romei C, et al. Post-Chernobyl thyroid carcinoma in Belarus children and adolescents: comparison with naturally occurring thyroid carcinoma in Italy and France. J Clin Endocrinol Metab. 1997; 82(11):3563–9.

18. Gharib H, Papini E. Thyroid nodules: clinical importance, assessment, and treatment. Endocrinol Metab Clin North Am. 2007; 36(3):707–35. vi.

19. Hall TL, Layfield LJ, Philippe A, Rosenthal DL. Sources of diagnostic error in fine needle aspiration of the thyroid. Cancer. 1989; 63(4):718–25.

20. Brander A, Viikinkoski P, Tuuhea J, Voutilainen L, Kivisaari L. Clinical versus ultrasound examination of the thyroid gland in common clinical practice. J Clin Ultrasound. 1992; 20(1):37–42.

21. Tan GH, Gharib H, Reading CC. Solitary thyroid nodule. Comparison between palpation and ultrasonography. Arch Intern Med. 1995; 155(22):2418–23.

22. Singh B, Shaha AR, Trivedi H, Carew JF, Poluri A, Shah JP. Coexistent Hashimoto's thyroiditis with papillary thyroid carcinoma: impact on presentation, management, and outcome. Surgery. 1999; 126(6):1070–6. ; discussion 6–7.

23. Repplinger D, Bargren A, Zhang YW, Adler JT, Haymart M, Chen H. Is Hashimoto's thyroiditis a risk factor for papillary thyroid cancer? J Surg Res. 2008; 150(1):49–52.

24. Boelaert K, Horacek J, Holder RL, Watkinson JC, Sheppard MC, Franklyn JA. Serum thyrotropin concentration as a novel predictor of malignancy in thyroid nodules investigated by fine-needle aspiration. J Clin Endocrinol Metab. 2006; 91(11):4295–301.

25. Haymart MR, Repplinger DJ, Leverson GE, Elson DF, Sippel RS, Jaume JC, et al. Higher serum thyroid stimulating hormone level in thyroid nodule patients is associated with greater risks of differentiated thyroid cancer and advanced tumor stage. J Clin Endocrinol Metab. 2008; 93(3):809–14.

26. Pacini F, Pinchera A, Giani C, Grasso L, Doveri F, Baschieri L. Serum thyroglobulin in thyroid carcinoma and other thyroid disorders. J Endocrinol Invest. 1980; 3(3):283–92.

27. Suh I, Vriens MR, Guerrero MA, Griffin A, Shen WT, Duh QY, et al. Serum thyroglobulin is a poor diagnostic biomarker of malignancy in follicular and Hurthle-cell neoplasms of the thyroid. Am J Surg. 2010; 200(1):41–6.
28. Lee EK, Chung KW, Min HS, Kim TS, Kim TH, Ryu JS, et al. Preoperative serum thyroglobulin as a useful predictive marker to differentiate follicular thyroid cancer from benign nodules in indeterminate nodules. J Korean Med Sci. 2012; 27(9):1014–8.

29. Elisei R, Bottici V, Luchetti F, Di Coscio G, Romei C, Grasso L, et al. Impact of routine measurement of serum calcitonin on the diagnosis and outcome of medullary thyroid cancer: experience in 10,864 patients with nodular thyroid disorders. J Clin Endocrinol Metab. 2004; 89(1):163–8.

30. Hahm JR, Lee MS, Min YK, Lee MK, Kim KW, Nam SJ, et al. Routine measurement of serum calcitonin is useful for early detection of medullary thyroid carcinoma in patients with nodular thyroid diseases. Thyroid. 2001; 11(1):73–80.

31. Niccoli P, Wion-Barbot N, Caron P, Henry JF, de Micco C, Saint Andre JP, et al. Interest of routine measurement of serum calcitonin: study in a large series of thyroidectomized patients. The French Medullary Study Group. J Clin Endocrinol Metab. 1997; 82(2):338–41.
32. Costante G, Meringolo D, Durante C, Bianchi D, Nocera M, Tumino S, et al. Predictive value of serum calcitonin levels for preoperative diagnosis of medullary thyroid carcinoma in a cohort of 5817 consecutive patients with thyroid nodules. J Clin Endocrinol Metab. 2007; 92(2):450–5.

33. Chambon G, Alovisetti C, Idoux-Louche C, Reynaud C, Rodier M, Guedj AM, et al. The use of preoperative routine measurement of basal serum thyrocalcitonin in candidates for thyroidectomy due to nodular thyroid disorders: results from 2733 consecutive patients. J Clin Endocrinol Metab. 2011; 96(1):75–81.

35. Karga H, Giagourta I, Papaioannou G, Doumouchtsis K, Polymeris A, Thanou S, et al. Changes in risk factors and Tumor Node Metastasis stage of sporadic medullary thyroid carcinoma over 41 years, before and after the routine measurements of serum calcitonin. Metabolism. 2011; 60(5):604–8.

36. Cheung K, Roman SA, Wang TS, Walker HD, Sosa JA. Calcitonin measurement in the evaluation of thyroid nodules in the United States: a cost-effectiveness and decision analysis. J Clin Endocrinol Metab. 2008; 93(6):2173–80.

37. Gagel RF, Hoff AO, Cote GJ. Medullary thyroid carcinoma. Braverman LE, Utiger D, editors. editors.Werner & Ingbar's the thyroid: a fundamental and clinical text. 9th ed.Philadelphia: Lippincott Williams & Wilkins;2005. p. 967–89.

38. Chen W, Parsons M, Torigian DA, Zhuang H, Alavi A. Evaluation of thyroid FDG uptake incidentally identified on FDG-PET/CT imaging. Nucl Med Commun. 2009; 30(3):240–4.

39. Nishimori H, Tabah R, Hickeson M, How J. Incidental thyroid "PETomas": clinical significance and novel description of the self-resolving variant of focal FDG-PET thyroid uptake. Can J Surg. 2011; 54(2):83–8.

40. Soelberg KK, Bonnema SJ, Brix TH, Hegedus L. Risk of malignancy in thyroid incidentalomas detected by 18Ffluorodeoxyglucose positron emission tomography: a systematic review. Thyroid. 2012; 22(9):918–25.

41. Frates MC, Benson CB, Charboneau JW, Cibas ES, Clark OH, Coleman BG, et al. Management of thyroid nodules detected at US: Society of Radiologists in Ultrasound consensus conference statement. Radiology. 2005; 237(3):794–800.

42. Papini E, Guglielmi R, Bianchini A, Crescenzi A, Taccogna S, Nardi F, et al. Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab. 2002; 87(5):1941–6.

43. Moon WJ, Jung SL, Lee JH, Na DG, Baek JH, Lee YH, et al. Benign and malignant thyroid nodules: US differentiation–multicenter retrospective study. Radiology. 2008; 247(3):762–70.

44. Brito JP, Gionfriddo MR, Al Nofal A, Boehmer KR, Leppin AL, Reading C, et al. The accuracy of thyroid nodule ultrasound to predict thyroid cancer: systematic review and metaanalysis. J Clin Endocrinol Metab. 2014; 99(4):1253–63.

45. Remonti LR, Kramer CK, Leitao CB, Pinto LC, Gross JL. Thyroid ultrasound features and risk of carcinoma: a systematic review and metaanalysis of observational studies. Thyroid. 2015; 25(5):538–50.

46. Campanella P, Ianni F, Rota CA, Corsello SM, Pontecorvi A. Quantification of cancer risk of each clinical and ultrasonographic suspicious feature of thyroid nodules: a systematic review and metaanalysis. Eur J Endocrinol. 2014; 170(5):R203–11.
47. Na DG, Baek JH, Sung JY, Kim JH, Kim JK, Choi YJ, et al. Thyroid imaging reporting and data system risk stratification of thyroid nodules: categorization based on solidity and echogenicity. Thyroid. 2016; 26(4):562–72.

48. Shin JH, Baek JH, Chung J, Ha EJ, Kim JH, Lee YH, et al. Ultrasonography diagnosis and imaging-based management of thyroid nodules: revised Korean Society of Thyroid Radiology consensus statement and recommendations. Korean J Radiol. 2016; 17(3):370–95.

49. Baloch ZW, LiVolsi VA, Asa SL, Rosai J, Merino MJ, Randolph G, et al. Diagnostic terminology and morphologic criteria for cytologic diagnosis of thyroid lesions: a synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference. Diagn Cytopathol. 2008; 36(6):425–37.

50. Crippa S, Mazzucchelli L, Cibas ES, Ali SZ. The Bethesda System for reporting thyroid fine-needle aspiration specimens. Am J Clin Pathol. 2010; 134(2):343–4. ; author reply 5.
51. Ali SZ, Cibas ES. The Bethesda system for reporting thyroid cytopathology. Definitions, criteria and explanatory notes. New York: Springer;2010. p. 1–166.
52. Bongiovanni M, Spitale A, Faquin WC, Mazzucchelli L, Baloch ZW. The Bethesda System for Reporting Thyroid Cytopathology: a metaanalysis. Acta Cytol. 2012; 56(4):333–9.

53. Theoharis CG, Schofield KM, Hammers L, Udelsman R, Chhieng DC. The Bethesda thyroid fine-needle aspiration classification system: year 1 at an academic institution. Thyroid. 2009; 19(11):1215–23.

54. Luu MH, Fischer AH, Pisharodi L, Owens CL. Improved preoperative definitive diagnosis of papillary thyroid carcinoma in FNAs prepared with both ThinPrep and conventional smears compared with FNAs prepared with ThinPrep alone. Cancer Cytopathol. 2011; 119(1):68–73.

55. Nayar R, Ivanovic M. The indeterminate thyroid fine-needle aspiration: experience from an academic center using terminology similar to that proposed in the 2007 National Cancer Institute Thyroid Fine Needle Aspiration State of the Science Conference. Cancer. 2009; 117(3):195–202.
56. Ohori NP, Schoedel KE. Variability in the atypia of undetermined significance/follicular lesion of undetermined significance diagnosis in the Bethesda System for Reporting Thyroid Cytopathology: sources and recommendations. Acta Cytol. 2011; 55(6):492–8.

57. Cibas ES, Baloch ZW, Fellegara G, LiVolsi VA, Raab SS, Rosai J, et al. A prospective assessment defining the limitations of thyroid nodule pathologic evaluation. Ann Intern Med. 2013; 159(5):325–32.

58. Nishino M, Wang HH. Should the thyroid AUS/FLUS category be further stratified by malignancy risk? Cancer Cytopathol. 2014; 122(7):481–3.

59. Park HJ, Moon JH, Yom CK, Kim KH, Choi JY, Choi SI, et al. Thyroid "atypia of undetermined significance" with nuclear atypia has high rates of malignancy and BRAF mutation. Cancer Cytopathol. 2014; 122(7):512–20.

60. Baloch ZW, Tam D, Langer J, Mandel S, LiVolsi VA, Gupta PK. Ultrasound-guided fine-needle aspiration biopsy of the thyroid: role of on-site assessment and multiple cytologic preparations. Diagn Cytopathol. 2000; 23(6):425–9.

61. Braga M, Cavalcanti TC, Collaco LM, Graf H. Efficacy of ultrasound-guided fine-needle aspiration biopsy in the diagnosis of complex thyroid nodules. J Clin Endocrinol Metab. 2001; 86(9):4089–91.

62. Redman R, Zalaznick H, Mazzaferri EL, Massoll NA. The impact of assessing specimen adequacy and number of needle passes for fine-needle aspiration biopsy of thyroid nodules. Thyroid. 2006; 16(1):55–60.

63. Orija IB, Pineyro M, Biscotti C, Reddy SS, Hamrahian AH. Value of repeating a nondiagnostic thyroid fine-needle aspiration biopsy. Endocr Pract. 2007; 13(7):735–42.

64. Wu HH, Rose C, Elsheikh TM. The Bethesda system for reporting thyroid cytopathology: An experience of 1,382 cases in a community practice setting with the implication for risk of neoplasm and risk of malignancy. Diagn Cytopathol. 2012; 40(5):399–403.
65. Layfield LJ, Abrams J, Cochand-Priollet B, Evans D, Gharib H, Greenspan F, et al. Post-thyroid FNA testing and treatment options: a synopsis of the National Cancer Institute Thyroid Fine Needle Aspiration State of the Science Conference. Diagn Cytopathol. 2008; 36(6):442–8.

66. Singh RS, Wang HH. Timing of repeat thyroid fine-needle aspiration in the management of thyroid nodules. Acta Cytol. 2011; 55(6):544–8.

67. Lubitz CC, Nagarkatti SS, Faquin WC, Samir AE, Hassan MC, Barbesino G, et al. Diagnostic yield of nondiagnostic thyroid nodules is not altered by timing of repeat biopsy. Thyroid. 2012; 22(6):590–4.

68. Alexander EK, Heering JP, Benson CB, Frates MC, Doubilet PM, Cibas ES, et al. Assessment of nondiagnostic ultrasound-guided fine needle aspirations of thyroid nodules. J Clin Endocrinol Metab. 2002; 87(11):4924–7.

69. Choi YS, Hong SW, Kwak JY, Moon HJ, Kim EK. Clinical and ultrasonographic findings affecting nondiagnostic results upon the second fine needle aspiration for thyroid nodules. Ann Surg Oncol. 2012; 19(7):2304–9.

70. Moon HJ, Kwak JY, Choi YS, Kim EK. How to manage thyroid nodules with two consecutive non-diagnostic results on ultrasonography-guided fine-needle aspiration. World J Surg. 2012; 36(3):586–92.

71. Na DG, Kim JH, Sung JY, Baek JH, Jung KC, Lee H, et al. Core-needle biopsy is more useful than repeat fine-needle aspiration in thyroid nodules read as nondiagnostic or atypia of undetermined significance by the Bethesda system for reporting thyroid cytopathology. Thyroid. 2012; 22(5):468–75.

72. Nam SY, Han BK, Ko EY, Kang SS, Hahn SY, Hwang JY, et al. BRAF V600E mutation analysis of thyroid nodules needle aspirates in relation to their ultrasongraphic classification: a potential guide for selection of samples for molecular analysis. Thyroid. 2010; 20(3):273–9.
73. Yip L, Nikiforova MN, Carty SE, Yim JH, Stang MT, Tublin MJ, et al. Optimizing surgical treatment of papillary thyroid carcinoma associated with BRAF mutation. Surgery. 2009; 146(6):1215–23.

74. Cantara S, Capezzone M, Marchisotta S, Capuano S, Busonero G, Toti P, et al. Impact of proto-oncogene mutation detection in cytological specimens from thyroid nodules improves the diagnostic accuracy of cytology. J Clin Endocrinol Metab. 2010; 95(3):1365–9.

75. Chehade JM, Silverberg AB, Kim J, Case C, Mooradian AD. Role of repeated fine-needle aspiration of thyroid nodules with benign cytologic features. Endocr Pract. 2001; 7(4):237–43.

76. Orlandi A, Puscar A, Capriata E, Fideleff H. Repeated fine-needle aspiration of the thyroid in benign nodular thyroid disease: critical evaluation of longterm follow-up. Thyroid. 2005; 15(3):274–8.

77. Oertel YC, Miyahara-Felipe L, Mendoza MG, Yu K. Value of repeated fine needle aspirations of the thyroid: an analysis of over ten thousand FNAs. Thyroid. 2007; 17(11):1061–6.

78. Erdogan MF, Kamel N, Aras D, Akdogan A, Baskal N, Erdogan G. Value of re-aspirations in benign nodular thyroid disease. Thyroid. 1998; 8(12):1087–90.

79. Illouz F, Rodien P, Saint-Andre JP, Triau S, Laboureau-Soares S, Dubois S, et al. Usefulness of repeated fine-needle cytology in the follow-up of non-operated thyroid nodules. Eur J Endocrinol. 2007; 156(3):303–8.

80. Tee YY, Lowe AJ, Brand CA, Judson RT. Fine-needle aspiration may miss a third of all malignancy in palpable thyroid nodules: a comprehensive literature review. Ann Surg. 2007; 246(5):714–20.
81. Pinchot SN, Al-Wagih H, Schaefer S, Sippel R, Chen H. Accuracy of fine-needle aspiration biopsy for predicting neoplasm or carcinoma in thyroid nodules 4 cm or larger. Arch Surg. 2009; 144(7):649–55.

82. Kuru B, Gulcelik NE, Gulcelik MA, Dincer H. The false-negative rate of fine-needle aspiration cytology for diagnosing thyroid carcinoma in thyroid nodules. Langenbecks Arch Surg. 2010; 395(2):127–32.

83. Wharry LI, McCoy KL, Stang MT, Armstrong MJ, LeBeau SO, Tublin ME, et al. Thyroid nodules (>/=4 cm): can ultrasound and cytology reliably exclude cancer? World J Surg. 2014; 38(3):614–21.
84. Yoon JH, Kwak JY, Moon HJ, Kim MJ, Kim EK. The diagnostic accuracy of ultrasound-guided fine-needle aspiration biopsy and the sonographic differences between benign and malignant thyroid nodules 3 cm or larger. Thyroid. 2011; 21(9):993–1000.
85. Porterfield JR Jr, Grant CS, Dean DS, Thompson GB, Farley DR, Richards ML, et al. Reliability of benign fine needle aspiration cytology of large thyroid nodules. Surgery. 2008; 144(6):963–8. ; discussion 8–9.

86. Nou E, Kwong N, Alexander LK, Cibas ES, Marqusee E, Alexander EK. Determination of the optimal time interval for repeat evaluation after a benign thyroid nodule aspiration. J Clin Endocrinol Metab. 2014; 99(2):510–6.

87. Bongiovanni M, Crippa S, Baloch Z, Piana S, Spitale A, Pagni F, et al. Comparison of 5-tiered and 6-tiered diagnostic systems for the reporting of thyroid cytopathology: a multiinstitutional study. Cancer Cytopathol. 2012; 120(2):117–25.
88. Davidov T, Trooskin SZ, Shanker BA, Yip D, Eng O, Crystal J, et al. Routine second-opinion cytopathology review of thyroid fine needle aspiration biopsies reduces diagnostic thyroidectomy. Surgery. 2010; 148(6):1294–9. ; discussion 9–301.

89. Kim SK, Hwang TS, Yoo YB, Han HS, Kim DL, Song KH, et al. Surgical results of thyroid nodules according to a management guideline based on the BRAF(V600E) mutation status. J Clin Endocrinol Metab. 2011; 96(3):658–64.

90. Adeniran AJ, Hui P, Chhieng DC, Prasad ML, Schofield K, Theoharis C. BRAF mutation testing of thyroid fine-needle aspiration specimens enhances the predictability of malignancy in thyroid follicular lesions of undetermined significance. Acta Cytol. 2011; 55(6):570–5.
91. Nikiforov YE, Ohori NP, Hodak SP, Carty SE, LeBeau SO, Ferris RL, et al. Impact of mutational testing on the diagnosis and management of patients with cytologically indeterminate thyroid nodules: a prospective analysis of 1056 FNA samples. J Clin Endocrinol Metab. 2011; 96(11):3390–7.

92. Nikiforov YE, Steward DL, Robinson-Smith TM, Haugen BR, Klopper JP, Zhu Z, et al. Molecular testing for mutations in improving the fine-needle aspiration diagnosis of thyroid nodules. J Clin Endocrinol Metab. 2009; 94(6):2092–8.

93. Alexander EK, Kennedy GC, Baloch ZW, Cibas ES, Chudova D, Diggans J, et al. Preoperative diagnosis of benign thyroid nodules with indeterminate cytology. N Engl J Med. 2012; 367(8):705–15.

94. Jeong SH, Hong HS, Lee EH, Cha JG, Park JS, Kwak JJ. Outcome of thyroid nodules characterized as atypia of undetermined significance or follicular lesion of undetermined significance and correlation with Ultrasound features and BRAF(V600E) mutation analysis. AJR Am J Roentgenol. 2013; 201(6):W854–60.

95. Yoo WS, Choi HS, Cho SW, Moon JH, Kim KW, Park HJ, et al. The role of ultrasound findings in the management of thyroid nodules with atypia or follicular lesions of undetermined significance. Clin Endocrinol (Oxf). 2014; 80(5):735–42.

96. Gweon HM, Son EJ, Youk JH, Kim JA. Thyroid nodules with Bethesda system III cytology: can ultrasonography guide the next step? Ann Surg Oncol. 2013; 20(9):3083–8.

97. Kim DW, Lee EJ, Jung SJ, Ryu JH, Kim YM. Role of sonographic diagnosis in managing Bethesda class III nodules. AJNR Am J Neuroradiol. 2011; 32(11):2136–41.

98. Nikiforov YE, Carty SE, Chiosea SI, Coyne C, Duvvuri U, Ferris RL, et al. Highly accurate diagnosis of cancer in thyroid nodules with follicular neoplasm/suspicious for a follicular neoplasm cytology by ThyroSeq v2 next-generation sequencing assay. Cancer. 2014; 120(23):3627–34.

99. Wang HH, Filie AC, Clark DP. Suspicious for malignancy. Ali SZ, Cibas ES, editors. editors.The Bethesda system for reporting thyroid cytopathology. New York: Springer;2010. p. 75–89.

100. Jara SM, Bhatnagar R, Guan H, Gocke CD, Ali SZ, Tufano RP. Utility of BRAF mutation detection in fine-needle aspiration biopsy samples read as "suspicious for papillary thyroid carcinoma". Head Neck. 2015; 37(12):1788–93.
101. Liu S, Gao A, Zhang B, Zhang Z, Zhao Y, Chen P, et al. Assessment of molecular testing in fine-needle aspiration biopsy samples: an experience in a Chinese population. Exp Mol Pathol. 2014; 97(2):292–7.

102. Hay ID. Management of patients with low-risk papillary thyroid carcinoma. Endocr Pract. 2007; 13(5):521–33.

103. Mazzaferri EL. Management of low-risk differentiated thyroid cancer. Endocr Pract. 2007; 13(5):498–512.

104. Bernet V, Hupart KH, Parangi S, Woeber KA. AACE/ACE disease state commentary: molecular diagnostic testing of thyroid nodules with indeterminate cytopathology. Endocr Pract. 2014; 20(4):360–3.

105. Ferris RL, Baloch Z, Bernet V, Chen A, Fahey TJ 3rd, Ganly I, et al. American Thyroid Association statement on surgical application of molecular profiling for thyroid nodules: current impact on perioperative decision making. Thyroid. 2015; 25(7):760–8.

106. Wang N, Zhai H, Lu Y. Is fluorine-18 fluorodeoxyglucose positron emission tomography useful for the thyroid nodules with indeterminate fine needle aspiration biopsy? A metaanalysis of the literature. J Otolaryngol Head Neck Surg. 2013; 42:38.

107. Vriens D, Adang EM, Netea-Maier RT, Smit JW, de Wilt JH, Oyen WJ, et al. Cost-effectiveness of FDG-PET/CT for cytologically indeterminate thyroid nodules: a decision analytic approach. J Clin Endocrinol Metab. 2014; 99(9):3263–74.

108. Deandreis D, Al Ghuzlan A, Auperin A, Vielh P, Caillou B, Chami L, et al. Is (18)F-fluorodeoxyglucose-PET/CT useful for the presurgical characterization of thyroid nodules with indeterminate fine needle aspiration cytology? Thyroid. 2012; 22(2):165–72.
109. Leenhardt L, Hejblum G, Franc B, Fediaevsky LD, Delbot T, Le Guillouzic D, et al. Indications and limits of ultrasound-guided cytology in the management of nonpalpable thyroid nodules. J Clin Endocrinol Metab. 1999; 84(1):24–8.

110. Carmeci C, Jeffrey RB, McDougall IR, Nowels KW, Weigel RJ. Ultrasound-guided fine-needle aspiration biopsy of thyroid masses. Thyroid. 1998; 8(4):283–9.

111. Ylagan LR, Farkas T, Dehner LP. Fine needle aspiration of the thyroid: a cytohistologic correlation and study of discrepant cases. Thyroid. 2004; 14(1):35–41.

112. Alexander EK, Hurwitz S, Heering JP, Benson CB, Frates MC, Doubilet PM, et al. Natural history of benign solid and cystic thyroid nodules. Ann Intern Med. 2003; 138(4):315–8.

113. Danese D, Sciacchitano S, Farsetti A, Andreoli M, Pontecorvi A. Diagnostic accuracy of conventional versus sonography-guided fine-needle aspiration biopsy of thyroid nodules. Thyroid. 1998; 8(1):15–21.

114. Papini E, Petrucci L, Guglielmi R, Panunzi C, Rinaldi R, Bacci V, et al. Long-term changes in nodular goiter: a 5-year prospective randomized trial of levothyroxine suppressive therapy for benign cold thyroid nodules. J Clin Endocrinol Metab. 1998; 83(3):780–3.

115. Brander AE, Viikinkoski VP, Nickels JI, Kivisaari LM. Importance of thyroid abnormalities detected at US screening: a 5-year follow-up. Radiology. 2000; 215(3):801–6.

116. Durante C, Costante G, Lucisano G, Bruno R, Meringolo D, Paciaroni A, et al. The natural history of benign thyroid nodules. JAMA. 2015; 313(9):926–35.

117. Zelmanovitz F, Genro S, Gross JL. Suppressive therapy with levothyroxine for solitary thyroid nodules: a double-blind controlled clinical study and cumulative metaanalyses. J Clin Endocrinol Metab. 1998; 83(11):3881–5.

118. Wemeau JL, Caron P, Schvartz C, Schlienger JL, Orgiazzi J, Cousty C, et al. Effects of thyroid-stimulating hormone suppression with levothyroxine in reducing the volume of solitary thyroid nodules and improving extranodular nonpalpable changes: a randomized, double-blind, placebocontrolled trial by the French Thyroid Research Group. J Clin Endocrinol Metab. 2002; 87(11):4928–34.
119. Castro MR, Caraballo PJ, Morris JC. Effectiveness of thyroid hormone suppressive therapy in benign solitary thyroid nodules: a metaanalysis. J Clin Endocrinol Metab. 2002; 87(9):4154–9.

120. Ahn JE, Lee JH, Yi JS, Shong YK, Hong SJ, Lee DH, et al. Diagnostic accuracy of CT and ultrasonography for evaluating metastatic cervical lymph nodes in patients with thyroid cancer. World J Surg. 2008; 32(7):1552–8.

121. Kim E, Park JS, Son KR, Kim JH, Jeon SJ, Na DG. Preoperative diagnosis of cervical metastatic lymph nodes in papillary thyroid carcinoma: comparison of ultrasound, computed tomography, and combined ultrasound with computed tomography. Thyroid. 2008; 18(4):411–8.

122. Lee DW, Ji YB, Sung ES, Park JS, Lee YJ, Park DW, et al. Roles of ultrasonography and computed tomography in the surgical management of cervical lymph node metastases in papillary thyroid carcinoma. Eur J Surg Oncol. 2013; 39(2):191–6.

123. Lesnik D, Cunnane ME, Zurakowski D, Acar GO, Ecevit C, Mace A, et al. Papillary thyroid carcinoma nodal surgery directed by a preoperative radiographic map utilizing CT scan and ultrasound in all primary and reoperative patients. Head Neck. 2014; 36(2):191–202.

124. Yeh MW, Bauer AJ, Bernet VA, Ferris RL, Loevner LA, Mandel SJ, et al. American Thyroid Association statement on preoperative imaging for thyroid cancer surgery. Thyroid. 2015; 25(1):3–14.

125. Solorzano CC, Carneiro DM, Ramirez M, Lee TM, Irvin GL 3rd. Surgeon-performed ultrasound in the management of thyroid malignancy. Am Surg. 2004; 70(7):576–80. ; discussion 80–2.
126. Shimamoto K, Satake H, Sawaki A, Ishigaki T, Funahashi H, Imai T. Preoperative staging of thyroid papillary carcinoma with ultrasonography. Eur J Radiol. 1998; 29(1):4–10.

127. Sivanandan R, Soo KC. Pattern of cervical lymph node metastases from papillary carcinoma of the thyroid. Br J Surg. 2001; 88(9):1241–4.

128. Ha EJ, Baek JH, Kim KW, Pyo J, Lee JH, Baek SH, et al. Comparative efficacy of radiofrequency and laser ablation for the treatment of benign thyroid nodules: systematic review including traditional pooling and bayesian network metaanalysis. J Clin Endocrinol Metab. 2015; 100(5):1903–11.

129. Baek JH, Ha EJ, Choi YJ, Sung JY, Kim JK, Shong YK. Radiofrequency versus ethanol ablation for treating predominantly cystic thyroid nodules: a randomized clinical trial. Korean J Radiol. 2015; 16(6):1332–40.

130. Sung JY, Baek JH, Kim KS, Lee D, Yoo H, Kim JK, et al. Single-session treatment of benign cystic thyroid nodules with ethanol versus radiofrequency ablation: a prospective randomized study. Radiology. 2013; 269(1):293–300.

131. Na DG, Lee JH, Jung SL, Kim JH, Sung JY, Shin JH, et al. Radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: consensus statement and recommendations. Korean J Radiol. 2012; 13(2):117–25.

132. Gharib H, Papini E, Paschke R, Duick DS, Valcavi R, Hegedus L, et al. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and EuropeanThyroid Association Medical Guidelines for clinical practice for the diagnosis and management of thyroid nodules. Endocr Pract. 2010; 16(Suppl 1):1–43.
133. Choi WJ, Baek JH, Choi YJ, Lee JH, Ha EJ, Lee WC, et al. Management of cystic or predominantly cystic thyroid nodules: role of simple aspiration of internal fluid. Endocr Res. 2015; 40(4):215–9.

134. Gharib H, Hegedus L, Pacella CM, Baek JH, Papini E. Clinical review: Nonsurgical, imageguided, minimally invasive therapy for thyroid nodules. J Clin Endocrinol Metab. 2013; 98(10):3949–57.
135. Leenhardt L, Erdogan MF, Hegedus L, Mandel SJ, Paschke R, Rago T, et al. 2013 European thyroid association guidelines for cervical ultrasound scan and ultrasound-guided techniques in the postoperative management of patients with thyroid cancer. Eur Thyroid J. 2013; 2(3):147–59.

136. Camargo R, Corigliano S, Friguglietti C, Gauna A, Harach R, Munizaga F, et al. Latin American thyroid society recommendations for the management of thyroid nodules. Arq Bras Endocrinol Metabol. 2009; 53(9):1167–75.

137. Sung JY, Baek JH, Jung SL, Kim JH, Kim KS, Lee D, et al. Radiofrequency ablation for autonomously functioning thyroid nodules: a multicenter study. Thyroid. 2015; 25(1):112–7.

138. Tan GH, Gharib H, Goellner JR, van Heerden JA, Bahn RS. Management of thyroid nodules in pregnancy. Arch Intern Med. 1996; 156(20):2317–20.

139. Kung AW, Chau MT, Lao TT, Tam SC, Low LC. The effect of pregnancy on thyroid nodule formation. J Clin Endocrinol Metab. 2002; 87(3):1010–4.

140. Mestman JH, Goodwin TM, Montoro MM. Thyroid disorders of pregnancy. Endocrinol Metab Clin North Am. 1995; 24(1):41–71.

141. Herzon FS, Morris DM, Segal MN, Rauch G, Parnell T. Coexistent thyroid cancer and pregnancy. Arch Otolaryngol Head Neck Surg. 1994; 120(11):1191–3.

142. Moosa M, Mazzaferri EL. Outcome of differentiated thyroid cancer diagnosed in pregnant women. J Clin Endocrinol Metab. 1997; 82(9):2862–6.

143. Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994; 97(5):418–28.

144. Rosen IB, Korman M, Walfish PG. Thyroid nodular disease in pregnancy: current diagnosis and management. Clin Obstet Gynecol. 1997; 40(1):81–9.

145. Aschebrook-Kilfoy B, Ward MH, Sabra MM, Devesa SS. Thyroid cancer incidence patterns in the United States by histologic type, 1992–2006. Thyroid. 2011; 21(2):125–34.

146. Hundahl SA, Fleming ID, Fremgen AM, Menck HR. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985–1995 [see commetns]. Cancer. 1998; 83(12):2638–48.
147. Hay ID, Bergstralh EJ, Goellner JR, Ebersold JR, Grant CS. Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery. 1993; 114(6):1050–7. ; discussion 7–8.
148. Shah MD, Hall FT, Eski SJ, Witterick IJ, Walfish PG, Freeman JL. Clinical course of thyroid carcinoma after neck dissection. Laryngoscope. 2003; 113(12):2102–7.

149. Wang TS, Dubner S, Sznyter LA, Heller KS. Incidence of metastatic well-differentiated thyroid cancer in cervical lymph nodes. Arch Otolaryngol Head Neck Surg. 2004; 130(1):110–3.

150. Mazzaferri EL. An overview of the management of papillary and follicular thyroid carcinoma. Thyroid. 1999; 9(5):421–7.

151. Mazzaferri EL. Long-term outcome of patients with differentiated thyroid carcinoma: effect of therapy. Endocr Pract. 2000; 6(6):469–76.

152. Cooper DS, Specker B, Ho M, Sperling M, Ladenson PW, Ross DS, et al. Thyrotropin suppression and disease progression in patients with differentiated thyroid cancer: results from the National Thyroid Cancer Treatment Cooperative Registry. Thyroid. 1998; 8(9):737–44.

153. Lin JD, Chao TC, Huang MJ, Weng HF, Tzen KY. Use of radioactive iodine for thyroid remnant ablation in well-differentiated thyroid carcinoma to replace thyroid reoperation. Am J Clin Oncol. 1998; 21(1):77–81.

154. Brierley JD, Panzarella T, Tsang RW, Gospodarowicz MK, O'Sullivan B. A comparison of different staging systems predictability of patient outcome. Thyroid carcinoma as an example. Cancer. 1997; 79(12):2414–23.
155. Hay ID, Thompson GB, Grant CS, Bergstralh EJ, Dvorak CE, Gorman CA, et al. Papillary thyroid carcinoma managed at the Mayo Clinic during six decades (1940–1999): temporal trends in initial therapy and longterm outcome in 2444 consecutively treated patients. World J Surg. 2002; 26(8):879–85.

156. Sosa JA, Bowman HM, Tielsch JM, Powe NR, Gordon TA, Udelsman R. The importance of surgeon experience for clinical and economic outcomes from thyroidectomy. Ann Surg. 1998; 228(3):320–30.

157. Loyo M, Tufano RP, Gourin CG. National trends in thyroid surgery and the effect of volume on short-term outcomes. Laryngoscope. 2013; 123(8):2056–63.

158. Stavrakis AI, Ituarte PH, Ko CY, Yeh MW. Surgeon volume as a predictor of outcomes in inpatient and outpatient endocrine surgery. Surgery. 2007; 142(6):887–99. ; discussion-99.

159. Qubain SW, Nakano S, Baba M, Takao S, Aikou T. Distribution of lymph node micrometastasis in pN0 welldifferentiated thyroid carcinoma. Surgery. 2002; 131(3):249–56.

160. Arturi F, Russo D, Giuffrida D, Ippolito A, Perrotti N, Vigneri R, et al. Early diagnosis by genetic analysis of differentiated thyroid cancer metastases in small lymph nodes. J Clin Endocrinol Metab. 1997; 82(5):1638–41.

161. Randolph GW, Duh QY, Heller KS, LiVolsi VA, Mandel SJ, Steward DL, et al. The prognostic significance of nodal metastases from papillary thyroid carcinoma can be stratified based on the size and number of metastatic lymph nodes, as well as the presence of extranodal extension. Thyroid. 2012; 22(11):1144–52.

162. Stulak JM, Grant CS, Farley DR, Thompson GB, van Heerden JA, Hay ID, et al. Value of preoperative ultrasonography in the surgical management of initial and reoperative papillary thyroid cancer. Arch Surg. 2006; 141(5):489–94. ; discussion 94–6.

163. Kouvaraki MA, Shapiro SE, Fornage BD, Edeiken-Monro BS, Sherman SI, Vassilopoulou-Sellin R, et al. Role of preoperative ultrasonography in the surgical management of patients with thyroid cancer. Surgery. 2003; 134(6):946–54. ; discussion 54–5.

164. O'Connell K, Yen TW, Quiroz F, Evans DB, Wang TS. The utility of routine preoperative cervical ultrasonography in patients undergoing thyroidectomy for differentiated thyroid cancer. Surgery. 2013; 154(4):697–701. ; discussion-3.
165. Leboulleux S, Girard E, Rose M, Travagli JP, Sabbah N, Caillou B, et al. Ultrasound criteria of malignancy for cervical lymph nodes in patients followed up for differentiated thyroid cancer. J Clin Endocrinol Metab. 2007; 92(9):3590–4.

166. Grani G, Fumarola A. Thyroglobulin in lymph node fine-needle aspiration washout: a systematic review and metaanalysis of diagnostic accuracy. J Clin Endocrinol Metab. 2014; 99(6):1970–82.

167. Pak K, Suh S, Hong H, Cheon GJ, Hahn SK, Kang KW, et al. Diagnostic values of thyroglobulin measurement in fine-needle aspiration of lymph nodes in patients with thyroid cancer. Endocrine. 2015; 49(1):70–7.

168. Duren M, Yavuz N, Bukey Y, Ozyegin MA, Gundogdu S, Acbay O, et al. Impact of initial surgical treatment on survival of patients with differentiated thyroid cancer: experience of an endocrine surgery center in an iodine-deficient region. World J Surg. 2000; 24(11):1290–4.

169. Stojadinovic A, Peoples GE, Libutti SK, Henry LR, Eberhardt J, Howard RS, et al. Development of a clinical decision model for thyroid nodules. BMC Surg. 2009; 9:12.

170. Tuttle RM, Lemar H, Burch HB. Clinical features associated with an increased risk of thyroid malignancy in patients with follicular neoplasia by fine-needle aspiration. Thyroid. 1998; 8(5):377–83.

171. Goldstein RE, Netterville JL, Burkey B, Johnson JE. Implications of follicular neoplasms, atypia, and lesions suspicious for malignancy diagnosed by fine-needle aspiration of thyroid nodules. Ann Surg. 2002; 235(5):656–62. ; discussion 62–4.

172. Schlinkert RT, van Heerden JA, Goellner JR, Gharib H, Smith SL, Rosales RF, et al. Factors that predict malignant thyroid lesions when fine-needle aspiration is "suspicious for follicular neoplasm". Mayo Clin Proc. 1997; 72(10):913–6.

173. Mehta RS, Carty SE, Ohori NP, Hodak SP, Coyne C, LeBeau SO, et al. Nodule size is an independent predictor of malignancy in mutation-negative nodules with follicular lesion of undetermined significance cytology. Surgery. 2013; 154(4):730–6. ; discussion 6–8.

174. Kandil E, Krishnan B, Noureldine SI, Yao L, Tufano RP. Hemithyroidectomy: a metaanalysis of postoperative need for hormone replacement and complications. ORL J Otorhinolaryngol Relat Spec. 2013; 75(1):6–17.

175. Verloop H, Louwerens M, Schoones JW, Kievit J, Smit JW, Dekkers OM. Risk of hypothyroidism following hemithyroi-dectomy: systematic review and metaanalysis of prognostic studies. J Clin Endocrinol Metab. 2012; 97(7):2243–55.

176. Gourin CG, Tufano RP, Forastiere AA, Koch WM, Pawlik TM, Bristow RE. Volume-based trends in thyroid surgery. Arch Otolaryngol Head Neck Surg. 2010; 136(12):1191–8.

177. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009; 19(11):1167–214.

178. Bilimoria KY, Bentrem DJ, Ko CY, Stewart AK, Winchester DP, Talamonti MS, et al. Extent of surgery affects survival for papillary thyroid cancer. Ann Surg. 2007; 246(3):375–81. ; discussion 81–4.

179. Grant CS, Hay ID, Gough IR, Bergstralh EJ, Goellner JR, McConahey WM. Local recurrence in papillary thyroid carcinoma: is extent of surgical resection important? Surgery. 1988; 104(6):954–62.
180. Hay ID, Grant CS, Bergstralh EJ, Thompson GB, van Heerden JA, Goellner JR. Unilateral total lobectomy: is it sufficient surgical treatment for patients with AMES low-risk papillary thyroid carcinoma? Surgery. 1998; 124(6):958–64. ; discussion 64–6.

181. Mazzaferri EL, Kloos RT. Clinical review 128: Current approaches to primary therapy for papillary and follicular thyroid cancer. J Clin Endocrinol Metab. 2001; 86(4):1447–63.
182. Matsuzu K, Sugino K, Masudo K, Nagahama M, Kitagawa W, Shibuya H, et al. Thyroid lobectomy for papillary thyroid cancer: longterm follow-up study of 1,088 cases. World J Surg. 2014; 38(1):68–79.

183. Barney BM, Hitchcock YJ, Sharma P, Shrieve DC, Tward JD. Overall and cause-specific survival for patients undergoing lobectomy, near-total, or total thyroidectomy for differentiated thyroid cancer. Head Neck. 2011; 33(5):645–9.

184. Mendelsohn AH, Elashoff DA, Abemayor E, St John MA. Surgery for papillary thyroid carcinoma: is lobectomy enough? Arch Otolaryngol Head Neck Surg. 2010; 136(11):1055–61.
185. Haigh PI, Urbach DR, Rotstein LE. Extent of thyroidectomy is not a major determinant of survival in low- or high-risk papillary thyroid cancer. Ann Surg Oncol. 2005; 12(1):81–9.

186. Nixon IJ, Ganly I, Patel SG, Palmer FL, Whitcher MM, Tuttle RM, et al. Thyroid lobectomy for treatment of well differentiated intrathyroid malignancy. Surgery. 2012; 151(4):571–9.

187. Adam MA, Pura J, Gu L, Dinan MA, Tyler DS, Reed SD, et al. Extent of surgery for papillary thyroid cancer is not associated with survival: an analysis of 61,775 patients. Ann Surg. 2014; 260(4):601–5. ; discussion 5–7.
188. Vaisman F, Shaha A, Fish S, Michael Tuttle R. Initial therapy with either thyroid lobectomy or total thyroidectomy without radioactive iodine remnant ablation is associated with very low rates of structural disease recurrence in properly selected patients with differentiated thyroid cancer. Clin Endocrinol (Oxf). 2011; 75(1):112–9.

189. Tuggle CT, Roman S, Udelsman R, Sosa JA. Same-day thyroidectomy: a review of practice patterns and outcomes for 1,168 procedures in New York State. Ann Surg Oncol. 2011; 18(4):1035–40.

190. Tuggle CT, Roman SA, Wang TS, Boudourakis L, Thomas DC, Udelsman R, et al. Pediatric endocrine surgery: who is operating on our children? Surgery. 2008; 144(6):869–77. ; discussion 77.

191. Kandil E, Noureldine SI, Abbas A, Tufano RP. The impact of surgical volume on patient outcomes following thyroid surgery. Surgery. 2013; 154(6):1346–52. ; discussion 52–3.

192. Hughes DT, White ML, Miller BS, Gauger PG, Burney RE, Doherty GM. Influence of prophylactic central lymph node dissection on postoperative thyroglobulin levels and radioiodine treatment in papillary thyroid cancer. Surgery. 2010; 148(6):1100–6. ; discussion 006–7.

193. Podnos YD, Smith D, Wagman LD, Ellenhorn JD. The implication of lymph node metastasis on survival in patients with well-differentiated thyroid cancer. Am Surg. 2005; 71(9):731–4.

194. Zaydfudim V, Feurer ID, Griffin MR, Phay JE. The impact of lymph node involvement on survival in patients with papillary and follicular thyroid carcinoma. Surgery. 2008; 144(6):1070–7. ; discussion 7–8.

195. Leboulleux S, Rubino C, Baudin E, Caillou B, Hartl DM, Bidart JM, et al. Prognostic factors for persistent or recurrent disease of papillary thyroid carcinoma with neck lymph node metastases and/or tumor extension beyond the thyroid capsule at initial diagnosis. J Clin Endocrinol Metab. 2005; 90(10):5723–9.

196. Sugitani I, Kasai N, Fujimoto Y, Yanagisawa A. A novel classification system for patients with PTC: addition of the new variables of large (3 cm or greater) nodal metastases and reclassification during the follow-up period. Surgery. 2004; 135(2):139–48.

197. Adam MA, Pura J, Goffredo P, Dinan MA, Reed SD, Scheri RP, et al. Presence and number of lymph node metastases are associated with compromised survival for patients younger than age 45 years with papillary thyroid cancer. J Clin Oncol. 2015; 33(21):2370–5.

198. Robbins KT, Shaha AR, Medina JE, Califano JA, Wolf GT, Ferlito A, et al. Consensus statement on the classification and terminology of neck dissection. Arch Otolaryngol Head Neck Surg. 2008; 134(5):536–8.

199. Hwang HS, Orloff LA. Efficacy of preoperative neck ultrasound in the detection of cervical lymph node metastasis from thyroid cancer. Laryngoscope. 2011; 121(3):487–91.

200. Mulla M, Schulte KM. Central cervical lymph node metastases in papillary thyroid cancer: a systematic review of imaging-guided and prophylactic removal of the central compartment. Clin Endocrinol (Oxf). 2012; 76(1):131–6.

201. Hartl DM, Leboulleux S, Al Ghuzlan A, Baudin E, Chami L, Schlumberger M, et al. Optimization of staging of the neck with prophylactic central and lateral neck dissection for papillary thyroid carcinoma. Ann Surg. 2012; 255(4):777–83.

202. Popadich A, Levin O, Lee JC, Smooke-Praw S, Ro K, Fazel M, et al. A multicenter cohort study of total thyroidectomy and routine central lymph node dissection for cN0 papillary thyroid cancer. Surgery. 2011; 150(6):1048–57.

203. Lang BH, Wong KP, Wan KY, Lo CY. Impact of routine unilateral central neck dissection on preablative and postablative stimulated thyroglobulin levels after total thyroidectomy in papillary thyroid carcinoma. Ann Surg Oncol. 2012; 19(1):60–7.

204. Wang TS, Evans DB, Fareau GG, Carroll T, Yen TW. Effect of prophylactic central compartment neck dissection on serum thyroglobulin and recommendations for adjuvant radioactive iodine in patients with differentiated thyroid cancer. Ann Surg Oncol. 2012; 19(13):4217–22.

205. American Thyroid Association Surgery Working Group, American Association of Endocrine Surgeons, American Academy of Otolaryngology-Head and Neck Surgery, American Head and Neck Society, Carty SE, Cooper DS, et al. Consensus statement on the terminology and classification of central neck dissection for thyroid cancer. Thyroid. 2009; 19(11):1153–8.

206. Chisholm EJ, Kulinskaya E, Tolley NS. Systematic review and metaanalysis of the adverse effects of thyroidectomy combined with central neck dissection as compared with thyroidectomy alone. Laryngoscope. 2009; 119(6):1135–9.

207. Bonnet S, Hartl D, Leboulleux S, Baudin E, Lumbroso JD, Al Ghuzlan A, et al. Prophylactic lymph node dissection for papillary thyroid cancer less than 2 cm: implications for radioiodine treatment. J Clin Endocrinol Metab. 2009; 94(4):1162–7.

208. Sancho JJ, Lennard TW, Paunovic I, Triponez F, Sitges-Serra A. Prophylactic central neck disection in papillary thyroid cancer: a consensus report of the European Society of Endocrine Surgeons (ESES). Langenbecks Arch Surg. 2014; 399(2):155–63.

209. Zetoune T, Keutgen X, Buitrago D, Aldailami H, Shao H, Mazumdar M, et al. Prophylactic central neck dissection and local recurrence in papillary thyroid cancer: a metaanalysis. Ann Surg Oncol. 2010; 17(12):3287–93.

210. Barczynski M, Konturek A, Stopa M, Nowak W. Prophylactic central neck dissection for papillary thyroid cancer. Br J Surg. 2013; 100(3):410–8.
211. Hartl DM, Mamelle E, Borget I, Leboulleux S, Mirghani H, Schlumberger M. Influence of prophylactic neck dissection on rate of retreatment for papillary thyroid carcinoma. World J Surg. 2013; 37(8):1951–8.

212. Sywak M, Cornford L, Roach P, Stalberg P, Sidhu S, Delbridge L. Routine ipsilateral level VI lymphadenectomy reduces postoperative thyroglobulin levels in papillary thyroid cancer. Surgery. 2006; 140(6):1000–5. ; discussion 5–7.

213. Laird AM, Gauger PG, Miller BS, Doherty GM. Evaluation of postoperative radioactive iodine scans in patients who underwent prophylactic central lymph node dissection. World J Surg. 2012; 36(6):1268–73.

214. Moreno MA, Edeiken-Monroe BS, Siegel ER, Sherman SI, Clayman GL. In papillary thyroid cancer, preoperative central neck ultrasound detects only macroscopic surgical disease, but negative findings predict excellent longterm regional control and survival. Thyroid. 2012; 22(4):347–55.

215. Yoo D, Ajmal S, Gowda S, Machan J, Monchik J, Mazzaglia P. Level VI lymph node dissection does not decrease radioiodine uptake in patients undergoing radioiodine ablation for differentiated thyroid cancer. World J Surg. 2012; 36(6):1255–61.

216. Roh JL, Park JY, Park CI. Total thyroidectomy plus neck dissection in differentiated papillary thyroid carcinoma patients: pattern of nodal metastasis, morbidity, recurrence, and postoperative levels of serum parathyroid hormone. Ann Surg. 2007; 245(4):604–10.
217. Cavicchi O, Piccin O, Caliceti U, De Cataldis A, Pasquali R, Ceroni AR. Transient hypoparathyroidism following thyroidectomy: a prospective study and multivariate analysis of 604 consecutive patients. Otolaryngol Head Neck Surg. 2007; 137(4):654–8.

218. Raffaelli M, De Crea C, Sessa L, Giustacchini P, Revelli L, Bellantone C, et al. Prospective evaluation of total thyroidectomy versus ipsilateral versus bilateral central neck dissection in patients with clinically node-negative papillary thyroid carcinoma. Surgery. 2012; 152(6):957–64.

219. Viola D, Materazzi G, Valerio L, Molinaro E, Agate L, Faviana P, et al. Prophylactic central compartment lymph node dissection in papillary thyroid carcinoma: clinical implications derived from the first prospective randomized controlled single institution study. J Clin Endocrinol Metab. 2015; 100(4):1316–24.

220. Lang BH, Ng SH, Lau LL, Cowling BJ, Wong KP, Wan KY. A systematic review and metaanalysis of prophylactic central neck dissection on short-term locoregional recurrence in papillary thyroid carcinoma after total thyroidectomy. Thyroid. 2013; 23(9):1087–98.

221. Wang TS, Cheung K, Farrokhyar F, Roman SA, Sosa JA. A metaanalysis of the effect of prophylactic central compartment neck dissection on locoregional recurrence rates in patients with papillary thyroid cancer. Ann Surg Oncol. 2013; 20(11):3477–83.

222. Gyorki DE, Untch B, Tuttle RM, Shaha AR. Prophylactic central neck dissection in differentiated thyroid cancer: an assessment of the evidence. Ann Surg Oncol. 2013; 20(7):2285–9.

223. Howell GM, Nikiforova MN, Carty SE, Armstrong MJ, Hodak SP, Stang MT, et al. BRAF V600E mutation independently predicts central compartment lymph node metastasis in patients with papillary thyroid cancer. Ann Surg Oncol. 2013; 20(1):47–52.

224. Xing M, Alzahrani AS, Carson KA, Viola D, Elisei R, Bendlova B, et al. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA. 2013; 309(14):1493–501.

225. Kim TH, Park YJ, Lim JA, Ahn HY, Lee EK, Lee YJ, et al. The association of the BRAF(V600E) mutation with prognostic factors and poor clinical outcome in papillary thyroid cancer: a metaanalysis. Cancer. 2012; 118(7):1764–73.
226. Ito Y, Yoshida H, Kihara M, Kobayashi K, Miya A, Miyauchi A. BRAF(V600E) mutation analysis in papillary thyroid carcinoma: is it useful for all patients? World J Surg. 2014; 38(3):679–87.

227. Gouveia C, Can NT, Bostrom A, Grenert JP, van Zante A, Orloff LA. Lack of association of BRAF mutation with negative prognostic indicators in papillary thyroid carcinoma: the University of California, San Francisco, experience. JAMA Otolaryngol Head Neck Surg. 2013; 139(11):1164–70.
228. Dutenhefner SE, Marui S, Santos AB, de Lima EU, Inoue M, Neto JS, et al. BRAF: a tool in the decision to perform elective neck dissection? Thyroid. 2013; 23(12):1541–6.

229. Lee KC, Li C, Schneider EB, Wang Y, Somervell H, Krafft M, et al. Is BRAF mutation associated with lymph node metastasis in patients with papillary thyroid cancer? Surgery. 2012; 152(6):977–83.

230. Xing M. Prognostic utility of BRAF mutation in papillary thyroid cancer. Mol Cell Endocrinol. 2010; 321(1):86–93.

231. Scheumann GF, Gimm O, Wegener G, Hundeshagen H, Dralle H. Prognostic significance and surgical management of locoregional lymph node metastases in papillary thyroid cancer. World J Surg. 1994; 18(4):559–67. ; discussion 67–8.

232. Sugitani I, Fujimoto Y, Yamada K, Yamamoto N. Prospective outcomes of selective lymph node dissection for papillary thyroid carcinoma based on preoperative ultrasonography. World J Surg. 2008; 32(11):2494–502.

233. Ito Y, Miyauchi A. Lateral and mediastinal lymph node dissection in differentiated thyroid carcinoma: indications, benefits, and risks. World J Surg. 2007; 31(5):905–15.

234. Gemsenjager E, Perren A, Seifert B, Schuler G, Schweizer I, Heitz PU. Lymph node surgery in papillary thyroid carcinoma. J Am Coll Surg. 2003; 197(2):182–90.
235. Kouvaraki MA, Lee JE, Shapiro SE, Sherman SI, Evans DB. Preventable reoperations for persistent and recurrent papillary thyroid carcinoma. Surgery. 2004; 136(6):1183–91.

236. Ito Y, Tomoda C, Uruno T, Takamura Y, Miya A, Kobayashi K, et al. Preoperative ultrasonographic examination for lymph node metastasis: usefulness when designing lymph node dissection for papillary microcarcinoma of the thyroid. World J Surg. 2004; 28(5):498–501.
237. Erdem E, Gulcelik MA, Kuru B, Alagol H. Comparison of completion thyroidectomy and primary surgery for differentiated thyroid carcinoma. Eur J Surg Oncol. 2003; 29(9):747–9.

238. Tan MP, Agarwal G, Reeve TS, Barraclough BH, Delbridge LW. Impact of timing on completion thyroidectomy for thyroid cancer. Br J Surg. 2002; 89(6):802–4.

239. Untch BR, Palmer FL, Ganly I, Patel SG, Michael Tuttle R, Shah JP, et al. Oncologic outcomes after completion thyroidectomy for patients with well-differentiated thyroid carcinoma. Ann Surg Oncol. 2014; 21(4):1374–8.

240. Husson O, Haak HR, Oranje WA, Mols F, Reemst PH, van de Poll-Franse LV. Health-related quality of life among thyroid cancer survivors: a systematic review. Clin Endocrinol (Oxf). 2011; 75(4):544–54.

241. Chandrasekhar SS, Randolph GW, Seidman MD, Rosenfeld RM, Angelos P, Barkmeier-Kraemer J, et al. Clinical practice guideline: improving voice outcomes after thyroid surgery. Otolaryngol Head Neck Surg. 2013; 148(6 Suppl):S1–37.
242. Rowe-Jones JM, Rosswick RP, Leighton SE. Benign thyroid disease and vocal cord palsy. Ann R Coll Surg Engl. 1993; 75(4):241–4.
243. Bergenfelz A, Jansson S, Kristoffersson A, Martensson H, Reihner E, Wallin G, et al. Complications to thyroid surgery: results as reported in a database from a multicenter audit comprising 3,660 patients. Langenbecks Arch Surg. 2008; 393(5):667–73.

244. Roh JL, Yoon YH, Park CI. Recurrent laryngeal nerve paralysis in patients with papillary thyroid carcinomas: evaluation and management of resulting vocal dysfunction. Am J Surg. 2009; 197(4):459–65.

245. Eadie TL, Kapsner M, Rosenzweig J, Waugh P, Hillel A, Merati A. The role of experience on judgments of dysphonia. J Voice. 2010; 24(5):564–73.

246. Jatzko GR, Lisborg PH, Muller MG, Wette VM. Recurrent nerve palsy after thyroid operations–principal nerve identification and a literature review. Surgery. 1994; 115(2):139–44.
247. Randolph GW, Dralle H, International Intraoperative Monitoring Study G, Abdullah H, Barczynski M, Bellantone R, et al. Electrophysiologic recurrent laryngeal nerve monitoring during thyroid and parathyroid surgery: international standards guideline statement. Laryngoscope. 2011; 121(Suppl 1):S1–16.

248. Pisanu A, Porceddu G, Podda M, Cois A, Uccheddu A. Systematic review with metaanalysis of studies comparing intraoperative neuromonitoring of recurrent laryngeal nerves versus visualization alone during thyroidectomy. J Surg Res. 2014; 188(1):152–61.

249. Goretzki PE, Schwarz K, Brinkmann J, Wirowski D, Lammers BJ. The impact of intraoperative neuromonitoring (IONM) on surgical strategy in bilateral thyroid diseases: is it worth the effort? World J Surg. 2010; 34(6):1274–84.

250. Barczynski M, Konturek A, Pragacz K, Papier A, Stopa M, Nowak W. Intraoperative nerve monitoring can reduce prevalence of recurrent laryngeal nerve injury in thyroid reoperations: results of a retrospective cohort study. World J Surg. 2014; 38(3):599–606.

251. Barczynski M, Konturek A, Cichon S. Randomized clinical trial of visualization versus neuromonitoring of recurrent laryngeal nerves during thyroidectomy. Br J Surg. 2009; 96(3):240–6.
252. Barczynski M, Konturek A, Stopa M, Honowska A, Nowak W. Randomized controlled trial of visualization versus neuromonitoring of the external branch of the superior laryngeal nerve during thyroidectomy. World J Surg. 2012; 36(6):1340–7.

253. Friedman AD, Burns JA, Heaton JT, Zeitels SM. Early versus late injection medialization for unilateral vocal cord paralysis. Laryngoscope. 2010; 120(10):2042–6.

254. Loh KC, Greenspan FS, Gee L, Miller TR, Yeo PP. Pathological tumor-node-metastasis (pTNM) staging for papillary and follicular thyroid carcinomas: a retrospective analysis of 700 patients. J Clin Endocrinol Metab. 1997; 82(11):3553–62.

255. DeGroot LJ, Kaplan EL, McCormick M, Straus FH. Natural history, treatment, and course of papillary thyroid carcinoma. J Clin Endocrinol Metab. 1990; 71(2):414–24.

256. Byar DP, Green SB, Dor P, Williams ED, Colon J, van Gilse HA, et al. A prognostic index for thyroid carcinoma. A study of the E.O.R.T.C. Thyroid Cancer Cooperative Group. Eur J Cancer. 1979; 15(8):1033–41.

257. Shaha AR, Loree TR, Shah JP. Prognostic factors and risk group analysis in follicular carcinoma of the thyroid. Surgery. 1995; 118(6):1131–6. ; discussion 6–8.

258. Sherman SI, Brierley JD, Sperling M, Ain KB, Bigos ST, Cooper DS, et al. Prospective multicenter study of thyroiscarcinoma treatment: initial analysis of staging and outcome. National Thyroid Cancer Treatment Cooperative Study Registry Group. Cancer. 1998; 83(5):1012–21.
259. Cady B, Rossi R. An expanded view of risk-group definition in differentiated thyroid carcinoma. Surgery. 1988; 104(6):947–53.
260. Tuttle RM, Tala H, Shah J, Leboeuf R, Ghossein R, Gonen M, et al. Estimating risk of recurrence in differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation: using response to therapy variables to modify the initial risk estimates predicted by the new American Thyroid Association staging system. Thyroid. 2010; 20(12):1341–9.

261. Tuttle RM, Leboeuf R. Follow up approaches in thyroid cancer: a risk adapted paradigm. Endocrinol Metab Clin North Am. 2008; 37(2):419–35. ix-x.

262. Momesso DP, Tuttle RM. Update on differentiated thyroid cancer staging. Endocrinol Metab Clin North Am. 2014; 43(2):401–21.

263. Vaisman F, Momesso D, Bulzico DA, Pessoa CH, Dias F, Corbo R, et al. Spontaneous remission in thyroid cancer patients after biochemical incomplete response to initial therapy. Clin Endocrinol (Oxf). 2012; 77(1):132–8.

264. Castagna MG, Maino F, Cipri C, Belardini V, Theodoropoulou A, Cevenini G, et al. Delayed risk stratification, to include the response to initial treatment (surgery and radioiodine ablation), has better outcome predictivity in differentiated thyroid cancer patients. Eur J Endocrinol. 2011; 165(3):441–6.

265. Berger F, Friedrich U, Knesewitsch P, Hahn K. Diagnostic 131I whole-body scintigraphy 1 year after thyroablative therapy in patients with differentiated thyroid cancer: correlation of results to the individual risk profile and longterm follow-up. Eur J Nucl Med Mol Imaging. 2011; 38(3):451–8.
266. Malandrino P, Latina A, Marescalco S, Spadaro A, Regalbuto C, Fulco RA, et al. Risk-adapted management of differentiated thyroid cancer assessed by a sensitive measurement of basal serum thyroglobulin. J Clin Endocrinol Metab. 2011; 96(6):1703–9.

267. Soyluk O, Boztepe H, Aral F, Alagol F, Ozbey NC. Papillary thyroid carcinoma patients assessed to be at low or intermediary risk after primary treatment are at greater risk of long term recurrence if they are thyroglobulin antibody positive or do not have distinctly low thyroglobulin at initial assessment. Thyroid. 2011; 21(12):1301–8.

268. Piccardo A, Arecco F, Morbelli S, Bianchi P, Barbera F, Finessi M, et al. Low thyroglobulin concentrations after thyroidectomy increase the prognostic value of undetectable thyroglobulin levels on levothyroxine suppressive treatment in low-risk differentiated thyroid cancer. J Endocrinol Invest. 2010; 33(2):83–7.

269. Castagna MG, Brilli L, Pilli T, Montanaro A, Cipri C, Fioravanti C, et al. Limited value of repeat recombinant human thyrotropin (rhTSH)-stimulated thyroglobulin testing in differentiated thyroid carcinoma patients with previous negative rhTSH-stimulated thyroglobulin and undetectable basal serum thyroglobulin levels. J Clin Endocrinol Metab. 2008; 93(1):76–81.

270. Kloos RT, Mazzaferri EL. A single recombinant human thyrotropin-stimulated serum thyroglobulin measurement predicts differentiated thyroid carcinoma metastases three to five years later. J Clin Endocrinol Metab. 2005; 90(9):5047–57.

271. Kloos RT. Thyroid cancer recurrence in patients clinically free of disease with undetectable or very low serum thyroglobulin values. J Clin Endocrinol Metab. 2010; 95(12):5241–8.

272. Han JM, Kim WB, Yim JH, Kim WG, Kim TY, Ryu JS, et al. Long-term clinical outcome of differentiated thyroid cancer patients with undetectable stimulated thyroglobulin level one year after initial treatment. Thyroid. 2012; 22(8):784–90.

273. Rosario PW, Furtado MS, Mineiro Filho AF, Lacerda RX, Calsolari MR. Value of repeat stimulated thyroglobulin testing in patients with differentiated thyroid carcinoma considered to be free of disease in the first year after ablation. Thyroid. 2012; 22(5):482–6.

274. Brassard M, Borget I, Edet-Sanson A, Giraudet AL, Mundler O, Toubeau M, et al. Long-term follow-up of patients with papillary and follicular thyroid cancer: a prospective study on 715 patients. J Clin Endocrinol Metab. 2011; 96(5):1352–9.

275. Pelttari H, Valimaki MJ, Loyttyniemi E, Schalin-Jantti C. Post-ablative serum thyroglobulin is an independent predictor of recurrence in low-risk differentiated thyroid carcinoma: a 16-year follow-up study. Eur J Endocrinol. 2010; 163(5):757–63.

276. Klubo-Gwiezdzinska J, Burman KD, Van Nostrand D, Wartofsky L. Does an undetectable rhTSH-stimulated Tg level 12 months after initial treatment of thyroid cancer indicate remission? Clin Endocrinol (Oxf). 2011; 74(1):111–7.
277. Crocetti U, Durante C, Attard M, Maniglia A, Tumino S, Bruno R, et al. Predictive value of recombinant human TSH stimulation and neck ultrasonography in differentiated thyroid cancer patients. Thyroid. 2008; 18(10):1049–53.

278. Torlontano M, Attard M, Crocetti U, Tumino S, Bruno R, Costante G, et al. Follow-up of low risk patients with papillary thyroid cancer: role of neck ultrasonography in detecting lymph node metastases. J Clin Endocrinol Metab. 2004; 89(7):3402–7.

279. Verburg FA, Stokkel MP, Duren C, Verkooijen RB, Mader U, van Isselt JW, et al. No survival difference after successful (131)I ablation between patients with initially low-risk and high-risk differentiated thyroid cancer. Eur J Nucl Med Mol Imaging. 2010; 37(2):276–83.

280. Giovanella L, Maffioli M, Ceriani L, De Palma D, Spriano G. Unstimulated high sensitive thyroglobulin measurement predicts outcome of differentiated thyroid carcinoma. Clin Chem Lab Med. 2009; 47(8):1001–4.

281. Lemb J, Hufner M, Meller B, Homayounfar K, Sahlmann C, Meller J. How reliable is secondary risk stratification with stimulated thyroglobulin in patients with differentiated thyroid carcinoma? Results from a retrospective study. Nuklearmedizin. 2013; 52(3):88–96.
282. Vaisman F, Tala H, Grewal R, Tuttle RM. In differentiated thyroid cancer, an incomplete structural response to therapy is associated with significantly worse clinical outcomes than only an incomplete thyroglobulin response. Thyroid. 2011; 21(12):1317–22.

283. Verburg FA, Luster M, Cupini C, Chiovato L, Duntas L, Elisei R, et al. Implications of thyroglobulin antibody positivity in patients with differentiated thyroid cancer: a clinical position statement. Thyroid. 2013; 23(10):1211–25.

284. Spencer CA, Takeuchi M, Kazarosyan M, Wang CC, Guttler RB, Singer PA, et al. Serum thyroglobulin autoantibodies: prevalence, influence on serum thyroglobulin measurement, and prognostic significance in patients with differentiated thyroid carcinoma. J Clin Endocrinol Metab. 1998; 83(4):1121–7.

285. Chung JK, Park YJ, Kim TY, So Y, Kim SK, Park DJ, et al. Clinical significance of elevated level of serum antithyroglobulin antibody in patients with differentiated thyroid cancer after thyroid ablation. Clin Endocrinol (Oxf). 2002; 57(2):215–21.

286. Gorges R, Maniecki M, Jentzen W, Sheu SN, Mann K, Bockisch A, et al. Development and clinical impact of thyroglobulin antibodies in patients with differentiated thyroid carcinoma during the first 3 years after thyroidectomy. Eur J Endocrinol. 2005; 153(1):49–55.

287. Seo JH, Lee SW, Ahn BC, Lee J. Recurrence detection in differentiated thyroid cancer patients with elevated serum level of antithyroglobulin antibody: special emphasis on using (18)F-FDG PET/CT. Clin Endocrinol (Oxf). 2010; 72(4):558–63.
288. Adil A, Jafri RA, Waqar A, Abbasi SA, Matiul H, Asghar AH, et al. Frequency and clinical importance of anti-Tg auto-antibodies (ATG). J Coll Physicians Surg Pak. 2003; 13(9):504–6.
289. Kim WG, Yoon JH, Kim WB, Kim TY, Kim EY, Kim JM, et al. Change of serum antithyroglobulin antibody levels is useful for prediction of clinical recurrence in thyroglobulinnegative patients with differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2008; 93(12):4683–9.

290. Chiovato L, Latrofa F, Braverman LE, Pacini F, Capezzone M, Masserini L, et al. Disappearance of humoral thyroid autoimmunity after complete removal of thyroid antigens. Ann Intern Med. 2003; 139(5 Pt 1):346–51.

291. Thomas D, Liakos V, Vassiliou E, Hatzimarkou F, Tsatsoulis A, Kaldrimides P. Possible reasons for different pattern disappearance of thyroglobulin and thyroid peroxidase autoantibodies in patients with differentiated thyroid carcinoma following total thyroidectomy and iodine-131 ablation. J Endocrinol Invest. 2007; 30(3):173–80.

292. Castagna MG, Tala Jury HP, Cipri C, Belardini V, Fioravanti C, Pasqui L, et al. The use of ultrasensitive thyroglobulin assays reduces but does not abolish the need for TSH stimulation in patients with differentiated thyroid carcinoma. J Endocrinol Invest. 2011; 34(8):e219–23.
293. Baudin E, Do Cao C, Cailleux AF, Leboulleux S, Travagli JP, Schlumberger M. Positive predictive value of serum thyroglobulin levels, measured during the first year of follow-up after thyroid hormone withdrawal, in thyroid cancer patients. J Clin Endocrinol Metab. 2003; 88(3):1107–11.

294. Pineda JD, Lee T, Ain K, Reynolds JC, Robbins J. Iodine-131 therapy for thyroid cancer patients with elevated thyroglobulin and negative diagnostic scan. J Clin Endocrinol Metab. 1995; 80(5):1488–92.

295. Alzahrani AS, Mohamed G, Al Shammary A, Aldasouqi S, Abdal Salam S, Shoukri M. Long-term course and predictive factors of elevated serum thyroglobulin and negative diagnostic radioiodine whole body scan in differentiated thyroid cancer. J Endocrinol Invest. 2005; 28(6):540–6.

296. Valadao MM, Rosario PW, Borges MA, Costa GB, Rezende LL, Padrao EL, et al. Positive predictive value of detectable stimulated tg during the first year after therapy of thyroid cancer and the value of comparison with Tg-ablation and Tg measured after 24 months. Thyroid. 2006; 16(11):1145–9.

297. Miyauchi A, Kudo T, Miya A, Kobayashi K, Ito Y, Takamura Y, et al. Prognostic impact of serum thyroglobulin doubling-time under thyrotropin suppression in patients with papillary thyroid carcinoma who underwent total thyroidectomy. Thyroid. 2011; 21(7):707–16.

298. Wong H, Wong KP, Yau T, Tang V, Leung R, Chiu J, et al. Is there a role for unstimulated thyroglobulin velocity in predicting recurrence in papillary thyroid carcinoma patients with detectable thyroglobulin after radioiodine ablation? Ann Surg Oncol. 2012; 19(11):3479–85.

299. Hsieh CJ, Wang PW. Sequential changes of serum antithyroglobulin antibody levels are a good predictor of disease activity in thyroglobulin-negative patients with papillary thyroid carcinoma. Thyroid. 2014; 24(3):488–93.

300. Schuff KG, Weber SM, Givi B, Samuels MH, Andersen PE, Cohen JI. Efficacy of nodal dissection for treatment of persistent/recurrent papillary thyroid cancer. Laryngoscope. 2008; 118(5):768–75.

301. Al-Saif O, Farrar WB, Bloomston M, Porter K, Ringel MD, Kloos RT. Long-term efficacy of lymph node reoperation for persistent papillary thyroid cancer. J Clin Endocrinol Metab. 2010; 95(5):2187–94.

302. Yim JH, Kim WB, Kim EY, Kim WG, Kim TY, Ryu JS, et al. The outcomes of first reoperation for locoregionally recurrent/persistent papillary thyroid carcinoma in patients who initially underwent total thyroidectomy and remnant ablation. J Clin Endocrinol Metab. 2011; 96(7):2049–56.

303. Chindris AM, Diehl NN, Crook JE, Fatourechi V, Smallridge RC. Undetectable sensitive serum thyroglobulin (<0.1 ng/ml) in 163 patients with follicular cell-derived thyroid cancer: results of rhTSH stimulation and neck ultrasonography and longterm biochemical and clinical follow-up. J Clin Endocrinol Metab. 2012; 97(8):2714–23.
304. Rondeau G, Fish S, Hann LE, Fagin JA, Tuttle RM. Ultrasonographically detected small thyroid bed nodules identified after total thyroidectomy for differentiated thyroid cancer seldom show clinically significant structural progression. Thyroid. 2011; 21(8):845–53.

305. Webb RC, Howard RS, Stojadinovic A, Gaitonde DY, Wallace MK, Ahmed J, et al. The utility of serum thyroglobulin measurement at the time of remnant ablation for predicting disease-free status in patients with differentiated thyroid cancer: a metaanalysis involving 3947 patients. J Clin Endocrinol Metab. 2012; 97(8):2754–63.

306. Giovanella L, Ceriani L, Suriano S, Ghelfo A, Maffioli M. Thyroglobulin measurement before rhTSH-aided 131I ablation in detecting metastases from differentiated thyroid carcinoma. Clin Endocrinol (Oxf). 2008; 69(4):659–63.
307. Giovanella L, Ceriani L, Ghelfo A, Keller F. Thyroglobulin assay 4 weeks after thyroidectomy predicts outcome in low-risk papillary thyroid carcinoma. Clin Chem Lab Med. 2005; 43(8):843–7.

308. Phan HT, Jager PL, van der Wal JE, Sluiter WJ, Plukker JT, Dierckx RA, et al. The follow-up of patients with differentiated thyroid cancer and undetectable thyroglobulin (Tg) and Tg antibodies during ablation. Eur J Endocrinol. 2008; 158(1):77–83.

309. Vaisman A, Orlov S, Yip J, Hu C, Lim T, Dowar M, et al. Application of postsurgical stimulated thyroglobulin for radioiodine remnant ablation selection in low-risk papillary thyroid carcinoma. Head Neck. 2010; 32(6):689–98.

310. Kim TY, Kim WB, Kim ES, Ryu JS, Yeo JS, Kim SC, et al. Serum thyroglobulin levels at the time of 131I remnant ablation just after thyroidectomy are useful for early prediction of clinical recurrence in low-risk patients with differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2005; 90(3):1440–5.
311. Toubeau M, Touzery C, Arveux P, Chaplain G, Vaillant G, Berriolo A, et al. Predictive value for disease progression of serum thyroglobulin levels measured in the postoperative period and after (131)I ablation therapy in patients with differentiated thyroid cancer. J Nucl Med. 2004; 45(6):988–94.
312. Piccardo A, Arecco F, Puntoni M, Foppiani L, Cabria M, Corvisieri S, et al. Focus on high-risk DTC patients: high postoperative serum thyroglobulin level is a strong predictor of disease persistence and is associated to progression-free survival and overall survival. Clin Nucl Med. 2013; 38(1):18–24.
313. Polachek A, Hirsch D, Tzvetov G, Grozinsky-Glasberg S, Slutski I, Singer J, et al. Prognostic value of post-thyroidectomy thyroglobulin levels in patients with differentiated thyroid cancer. J Endocrinol Invest. 2011; 34(11):855–60.
314. Heemstra KA, Liu YY, Stokkel M, Kievit J, Corssmit E, Pereira AM, et al. Serum thyroglobulin concentrations predict disease-free remission and death in differentiated thyroid carcinoma. Clin Endocrinol (Oxf). 2007; 66(1):58–64.

315. Lin JD, Huang MJ, Hsu BR, Chao TC, Hsueh C, Liu FH, et al. Significance of postoperative serum thyroglobulin levels in patients with papillary and follicular thyroid carcinomas. J Surg Oncol. 2002; 80(1):45–51.

316. Lee JI, Chung YJ, Cho BY, Chong S, Seok JW, Park SJ. Postoperative-stimulated serum thyroglobulin measured at the time of 131I ablation is useful for the prediction of disease status in patients with differentiated thyroid carcinoma. Surgery. 2013; 153(6):828–35.
317. Lepoutre-Lussey C, Maddah D, Golmard JL, Russ G, Tissier F, Tresallet C, et al. Post-operative neck ultrasound and risk stratification in differentiated thyroid cancer patients with initial lymph node involvement. Eur J Endocrinol. 2014; 170(6):837–46.

318. Avram AM, Fig LM, Frey KA, Gross MD, Wong KK. Preablation 131-I scans with SPECT/CT in postoperative thyroid cancer patients: what is the impact on staging? J Clin Endocrinol Metab. 2013; 98(3):1163–71.

319. Chen MK, Yasrebi M, Samii J, Staib LH, Doddamane I, Cheng DW. The utility of I-123 pretherapy scan in I-131 radioiodine therapy for thyroid cancer. Thyroid. 2012; 22(3):304–9.

320. Van Nostrand D, Aiken M, Atkins F, Moreau S, Garcia C, Acio E, et al. The utility of radioiodine scans prior to iodine 131 ablation in patients with well-differentiated thyroid cancer. Thyroid. 2009; 19(8):849–55.

321. Gerard SK, Cavalieri RR. I-123 diagnostic thyroid tumor whole-body scanning with imaging at 6, 24, and 48 hours. Clin Nucl Med. 2002; 27(1):1–8.

322. Chakravarty D, Santos E, Ryder M, Knauf JA, Liao XH, West BL, et al. Small-molecule MAPK inhibitors restore radioiodine incorporation in mouse thyroid cancers with conditional BRAF activation. J Clin Invest. 2011; 121(12):4700–11.

323. Schvartz C, Bonnetain F, Dabakuyo S, Gauthier M, Cueff A, Fieffe S, et al. Impact on overall survival of radioactive iodine in low-risk differentiated thyroid cancer patients. J Clin Endocrinol Metab. 2012; 97(5):1526–35.

324. Jonklaas J, Sarlis NJ, Litofsky D, Ain KB, Bigos ST, Brierley JD, et al. Outcomes of patients with differentiated thyroid carcinoma following initial therapy. Thyroid. 2006; 16(12):1229–42.

325. Jonklaas J, Cooper DS, Ain KB, Bigos T, Brierley JD, Haugen BR, et al. Radioiodine therapy in patients with stage I differentiated thyroid cancer. Thyroid. 2010; 20(12):1423–4.

326. Lamartina L, Durante C, Filetti S, Cooper DS. Low-risk differentiated thyroid cancer and radioiodine remnant ablation: a systematic review of the literature. J Clin Endocrinol Metab. 2015; 100(5):1748–61.

327. Podnos YD, Smith DD, Wagman LD, Ellenhorn JD. Survival in patients with papillary thyroid cancer is not affected by the use of radioactive isotope. J Surg Oncol. 2007; 96(1):3–7.

328. Mallick U, Harmer C, Yap B, Wadsley J, Clarke S, Moss L, et al. Ablation with low-dose radioiodine and thyrotropin alfa in thyroid cancer. N Engl J Med. 2012; 366(18):1674–85.

329. Schlumberger M, Catargi B, Borget I, Deandreis D, Zerdoud S, Bridji B, et al. Strategies of radioiodine ablation in patients with low-risk thyroid cancer. N Engl J Med. 2012; 366(18):1663–73.

330. Ha S, Oh SW, Kim YK, Koo do H, Jung YH, Yi KH, et al. Clinical outcome of remnant thyroid ablation with low dose radioiodine in korean patients with low to intermediate-risk thyroid cancer. J Korean Med Sci. 2015; 30(7):876–81.

331. Sohn SY, Choi JY, Jang HW, Kim HJ, Jin SM, Kim SW, et al. Association between excessive urinary iodine excretion and failure of radioactive iodine thyroid ablation in patients with papillary thyroid cancer. Thyroid. 2013; 23(6):741–7.

332. Verburg FA, Aktolun C, Chiti A, Frangos S, Giovanella L, Hoffmann M, et al. Why the European Association of Nuclear Medicine has declined to endorse the 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer. Eur J Nucl Med Mol Imaging. 2016; 43(6):1001–5.

333. Blumhardt R, Wolin EA, Phillips WT, Salman UA, Walker RC, Stack BC Jr, et al. Current controversies in the initial postsurgical radioactive iodine therapy for thyroid cancer: a narrative review. Endocr Relat Cancer. 2014; 21(6):R473–84.

334. Edmonds CJ, Hayes S, Kermode JC, Thompson BD. Measurement of serum TSH and thyroid hormones in the management of treatment of thyroid carcinoma with radioiodine. Br J Radiol. 1977; 50(599):799–807.

335. Torres MS, Ramirez L, Simkin PH, Braverman LE, Emerson CH. Effect of various doses of recombinant human thyrotropin on the thyroid radioactive iodine uptake and serum levels of thyroid hormones and thyroglobulin in normal subjects. J Clin Endocrinol Metab. 2001; 86(4):1660–4.

336. Hershman JM, Edwards CL. Serum thyrotropin (TSH) levels after thyroid ablation compared with TSH levels after exogenous bovine TSH: implications for 131-I treatment of thyroid carcinoma. J Clin Endocrinol Metab. 1972; 34(5):814–8.
337. Hilts SV, Hellman D, Anderson J, Woolfenden J, Van Antwerp J, Patton D. Serial TSH determination after T3 withdrawal or thyroidectomy in the therapy of thyroid carcinoma. J Nucl Med. 1979; 20(9):928–32.
338. Martin ND. Endogenous serum TSH levels and metastatic survey scans in thyroid cancer patients using triiodothyronine withdrawal. Clin Nucl Med. 1978; 3(10):401–3.

339. Goldman JM, Line BR, Aamodt RL, Robbins J. Influence of triiodothyronine withdrawal time on 131I uptake postthyroi-dectomy for thyroid cancer. J Clin Endocrinol Metab. 1980; 50(4):734–9.
340. Schneider AB, Line BR, Goldman JM, Robbins J. Sequential serum thyroglobulin determinations, 131I scans, and 131I uptakes after triiodothyronine withdrawal in patients with thyroid cancer. J Clin Endocrinol Metab. 1981; 53(6):1199–206.
341. Maxon HR, Thomas SR, Hertzberg VS, Kereiakes JG, Chen IW, Sperling MI, et al. Relation between effective radiation dose and outcome of radioiodine therapy for thyroid cancer. N Engl J Med. 1983; 309(16):937–41.

342. Liel Y. Preparation for radioactive iodine administration in differentiated thyroid cancer patients. Clin Endocrinol (Oxf). 2002; 57(4):523–7.

343. Sanchez R, Espinosa-de-los-Monteros AL, Mendoza V, Brea E, Hernandez I, Sosa E, et al. Adequate thyroid-stimulating hormone levels after levothyroxine discontinuation in the follow-up of patients with well-differentiated thyroid carcinoma. Arch Med Res. 2002; 33(5):478–81.
344. Grigsby PW, Siegel BA, Bekker S, Clutter WE, Moley JF. Preparation of patients with thyroid cancer for 131I scintigraphy or therapy by 1–3 weeks of thyroxine discontinuation. J Nucl Med. 2004; 45(4):567–70.
345. Serhal DI, Nasrallah MP, Arafah BM. Rapid rise in serum thyrotropin concentrations after thyroidectomy or withdrawal of suppressive thyroxine therapy in preparation for radioactive iodine administration to patients with differentiated thyroid cancer. J Clin Endocrinol Metab. 2004; 89(7):3285–9.

346. Lee J, Yun MJ, Nam KH, Chung WY, Soh EY, Park CS. Quality of life and effectiveness comparisons of thyroxine withdrawal, triiodothyronine withdrawal, and recombinant thyroid-stimulating hormone administration for low-dose radioiodine remnant ablation of differentiated thyroid carcinoma. Thyroid. 2010; 20(2):173–9.

347. Leboeuf R, Perron P, Carpentier AC, Verreault J, Langlois MF. L-T3 preparation for whole-body scintigraphy: a randomized-controlled trial. Clin Endocrinol (Oxf). 2007; 67(6):839–44.
348. Fallahi B, Beiki D, Takavar A, Fard-Esfahani A, Gilani KA, Saghari M, et al. Low versus high radioiodine dose in postoperative ablation of residual thyroid tissue in patients with differentiated thyroid carcinoma: a large randomized clinical trial. Nucl Med Commun. 2012; 33(3):275–82.
349. Prpic M, Dabelic N, Stanicic J, Jukic T, Milosevic M, Kusic Z. Adjuvant thyroid remnant ablation in patients with differentiated thyroid carcinoma confined to the thyroid: a comparison of ablation success with different activities of radioiodine (I-131). Ann Nucl Med. 2012; 26(9):744–51.

350. Karam M, Gianoukakis A, Feustel PJ, Cheema A, Postal ES, Cooper JA. Influence of diagnostic and therapeutic doses on thyroid remnant ablation rates. Nucl Med Commun. 2003; 24(5):489–95.

351. Robbins RJ, Driedger A, Magner J, U.S, Canadian Thyrogen Compassionate Use Program Investigator Group. Recombinant human thyrotropin-assisted radioiodine therapy for patients with metastatic thyroid cancer who could not elevate endogenous thyrotropin or be withdrawn from thyroxine. Thyroid. 2006; 16(11):1121–30.
352. Pacini F, Ladenson PW, Schlumberger M, Driedger A, Luster M, Kloos RT, et al. Radioiodine ablation of thyroid remnants after preparation with recombinant human thyrotropin in differentiated thyroid carcinoma: results of an international, randomized, controlled study. J Clin Endocrinol Metab. 2006; 91(3):926–32.

353. Maxon HR, Thomas SR, Boehringer A, Drilling J, Sperling MI, Sparks JC, et al. Low iodine diet in I-131 ablation of thyroid remnants. Clin Nucl Med. 1983; 8(3):123–6.

354. Goslings BM. Proceedings: Effect of a low iodine diet on 131-I therapy in follicular thyroid carcinomata. J Endocrinol. 1975; 64(3):): 30P.
355. Pluijmen MJ, Eustatia-Rutten C, Goslings BM, Stokkel MP, Arias AM, Diamant M, et al. Effects of low-iodide diet on postsurgical radioiodide ablation therapy in patients with differentiated thyroid carcinoma. Clin Endocrinol (Oxf). 2003; 58(4):428–35.

356. Park JT 2nd, Hennessey JV. Two-week low iodine diet is necessary for adequate outpatient preparation for radioiodine rhTSH scanning in patients taking levothyroxine. Thyroid. 2004; 14(1):57–63.

357. Tomoda C, Uruno T, Takamura Y, Ito Y, Miya A, Kobayashi K, et al. Reevaluation of stringent low iodine diet in outpatient preparation for radioiodine examination and therapy. Endocr J. 2005; 52(2):237–40.

358. Morris LF, Wilder MS, Waxman AD, Braunstein GD. Reevaluation of the impact of a stringent low-iodine diet on ablation rates in radioiodine treatment of thyroid carcinoma. Thyroid. 2001; 11(8):749–55.

359. Fatourechi V, Hay ID, Mullan BP, Wiseman GA, Eghbali-Fatourechi GZ, Thorson LM, et al. Are posttherapy radioiodine scans informative and do they influence subsequent therapy of patients with differentiated thyroid cancer? Thyroid. 2000; 10(7):573–7.

360. Sherman SI, Tielens ET, Sostre S, Wharam MD Jr, Ladenson PW. Clinical utility of posttreatment radioiodine scans in the management of patients with thyroid carcinoma. J Clin Endocrinol Metab. 1994; 78(3):629–34.

361. Souza Rosario PW, Barroso AL, Rezende LL, Padrao EL, Fagundes TA, Penna GC, et al. Post I-131 therapy scanning in patients with thyroid carcinoma metastases: an unnecessary cost or a relevant contribution? Clin Nucl Med. 2004; 29(12):795–8.
362. Ciappuccini R, Heutte N, Trzepla G, Rame JP, Vaur D, Aide N, et al. Postablation (131)I scintigraphy with neck and thorax SPECT-CT and stimulated serum thyroglobulin level predict the outcome of patients with differentiated thyroid cancer. Eur J Endocrinol. 2011; 164(6):961–9.

363. Kohlfuerst S, Igerc I, Lobnig M, Gallowitsch HJ, Gomez-Segovia I, Matschnig S, et al. Posttherapeutic (131)I SPECT-CT offers high diagnostic accuracy when the findings on conventional planar imaging are inconclusive and allows a tailored patient treatment regimen. Eur J Nucl Med Mol Imaging. 2009; 36(6):886–93.

364. Chen L, Luo Q, Shen Y, Yu Y, Yuan Z, Lu H, et al. Incremental value of 131I SPECT/CT in the management of patients with differentiated thyroid carcinoma. J Nucl Med. 2008; 49(12):1952–7.

365. Schmidt D, Linke R, Uder M, Kuwert T. Five months' follow-up of patients with and without iodine-positive lymph node metastases of thyroid carcinoma as disclosed by (131)I-SPECT/CT at the first radioablation. Eur J Nucl Med Mol Imaging. 2010; 37(4):699–705.

366. Maruoka Y, Abe K, Baba S, Isoda T, Sawamoto H, Tanabe Y, et al. Incremental diagnostic value of SPECT/CT with 131I scintigraphy after radioiodine therapy in patients with well-differentiated thyroid carcinoma. Radiology. 2012; 265(3):902–9.
367. Grewal RK, Tuttle RM, Fox J, Borkar S, Chou JF, Gonen M, et al. The effect of posttherapy 131I SPECT/CT on risk classification and management of patients with differentiated thyroid cancer. J Nucl Med. 2010; 51(9):1361–7.

368. Brabant G. Thyrotropin suppressive therapy in thyroid carcinoma: what are the targets? J Clin Endocrinol Metab. 2008; 93(4):1167–9.

369. Diessl S, Holzberger B, Mader U, Grelle I, Smit JW, Buck AK, et al. Impact of moderate vs stringent TSH suppression on survival in advanced differentiated thyroid carcinoma. Clin Endocrinol (Oxf). 2012; 76(4):586–92.

370. McGriff NJ, Csako G, Gourgiotis L, Lori CG, Pucino F, Sarlis NJ. Effects of thyroid hormone suppression therapy on adverse clinical outcomes in thyroid cancer. Ann Med. 2002; 34(7–8):554–64.

371. Pujol P, Daures JP, Nsakala N, Baldet L, Bringer J, Jaffiol C. Degree of thyrotropin suppression as a prognostic determinant in differentiated thyroid cancer. J Clin Endocrinol Metab. 1996; 81(12):4318–23.

372. Wang PW, Wang ST, Liu RT, Chien WY, Tung SC, Lu YC, et al. Levothyroxine suppression of thyroglobulin in patients with differentiated thyroid carcinoma. J Clin Endocrinol Metab. 1999; 84(12):4549–53.

373. Toft AD. Clinical practice. Subclinical hyperthyroidism. N Engl J Med. 2001; 345(7):512–6.
374. Ebina A, Sugitani I, Fujimoto Y, Yamada K. Risk-adapted management of papillary thyroid carcinoma according to our own risk group classification system: is thyroid lobectomy the treatment of choice for low-risk patients? Surgery. 2014; 156(6):1579–88. ; discussion 88–9.
375. Ford D, Giridharan S, McConkey C, Hartley A, Brammer C, Watkinson JC, et al. External beam radiotherapy in the management of differentiated thyroid cancer. Clin Oncol (R Coll Radiol). 2003; 15(6):337–41.

376. Terezakis SA, Lee KS, Ghossein RA, Rivera M, Tuttle RM, Wolden SL, et al. Role of external beam radiotherapy in patients with advanced or recurrent nonanaplastic thyroid cancer: Memorial Sloan-kettering Cancer Center experience. Int J Radiat Oncol Biol Phys. 2009; 73(3):795–801.

377. Links TP, van Tol KM, Jager PL, Plukker JT, Piers DA, Boezen HM, et al. Life expectancy in differentiated thyroid cancer: a novel approach to survival analysis. Endocr Relat Cancer. 2005; 12(2):273–80.

378. Kim S, Wei JP, Braveman JM, Brams DM. Predicting outcome and directing therapy for papillary thyroid carcinoma. Arch Surg. 2004; 139(4):390–4. ; discussion 3–4.

379. Eustatia-Rutten CF, Smit JW, Romijn JA, van der Kleij-Corssmit EP, Pereira AM, Stokkel MP, et al. Diagnostic value of serum thyroglobulin measurements in the follow-up of differentiated thyroid carcinoma, a structured metaanalysis. Clin Endocrinol (Oxf). 2004; 61(1):61–74.

380. Schlumberger M, Berg G, Cohen O, Duntas L, Jamar F, Jarzab B, et al. Follow-up of low-risk patients with differentiated thyroid carcinoma: a European perspective. Eur J Endocrinol. 2004; 150(2):105–12.

381. Bachelot A, Leboulleux S, Baudin E, Hartl DM, Caillou B, Travagli JP, et al. Neck recurrence from thyroid carcinoma: serum thyroglobulin and high-dose total body scan are not reliable criteria for cure after radioiodine treatment. Clin Endocrinol (Oxf). 2005; 62(3):376–9.

382. Brierley JD, Tsang RW. External-beam radiation therapy in the treatment of differentiated thyroid cancer. Semin Surg Oncol. 1999; 16(1):42–9.

383. Ronga G, Filesi M, Montesano T, Di Nicola AD, Pace C, Travascio L, et al. Lung metastases from differentiated thyroid carcinoma. A 40 years' experience. Q J Nucl Med Mol Imaging. 2004; 48(1):12–9.
384. Schlumberger M, Challeton C, De Vathaire F, Travagli JP, Gardet P, Lumbroso JD, et al. Radioactive iodine treatment and external radiotherapy for lung and bone metastases from thyroid carcinoma. J Nucl Med. 1996; 37(4):598–605.
385. Ilgan S, Karacalioglu AO, Pabuscu Y, Atac GK, Arslan N, Ozturk E, et al. Iodine-131 treatment and high-resolution CT: results in patients with lung metastases from differentiated thyroid carcinoma. Eur J Nucl Med Mol Imaging. 2004; 31(6):825–30.

386. Bernier MO, Leenhardt L, Hoang C, Aurengo A, Mary JY, Menegaux F, et al. Survival and therapeutic modalities in patients with bone metastases of differentiated thyroid carcinomas. J Clin Endocrinol Metab. 2001; 86(4):1568–73.

387. Zettinig G, Fueger BJ, Passler C, Kaserer K, Pirich C, Dudczak R, et al. Long-term follow-up of patients with bone metastases from differentiated thyroid carcinoma – surgery or conventional therapy? Clin Endocrinol (Oxf). 2002; 56(3):377–82.

388. Spencer CA, LoPresti JS, Fatemi S, Nicoloff JT. Detection of residual and recurrent differentiated thyroid carcinoma by serum thyroglobulin measurement. Thyroid. 1999; 9(5):435–41.

389. Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002; 87(2):489–99.

390. Spencer CA. Challenges of serum thyroglobulin (Tg) measurement in the presence of Tg autoantibodies. J Clin Endocrinol Metab. 2004; 89(8):3702–4.

391. Bachelot A, Cailleux AF, Klain M, Baudin E, Ricard M, Bellon N, et al. Relationship between tumor burden and serum thyroglobulin level in patients with papillary and follicular thyroid carcinoma. Thyroid. 2002; 12(8):707–11.

392. David A, Blotta A, Rossi R, Zatelli MC, Bondanelli M, Roti E, et al. Clinical value of different responses of serum thyroglobulin to recombinant human thyrotropin in the follow-up of patients with differentiated thyroid carcinoma. Thyroid. 2005; 15(3):267–73.

393. Schaap J, Eustatia-Rutten CF, Stokkel M, Links TP, Diamant M, van der Velde EA, et al. Does radioiodine therapy have disadvantageous effects in non-iodine accumulating differentiated thyroid carcinoma? Clin Endocrinol (Oxf). 2002; 57(1):117–24.

394. Spencer CA. Clinical review: Clinical utility of thyroglobulin antibody (TgAb) measurements for patients with differentiated thyroid cancers (DTC). J Clin Endocrinol Metab. 2011; 96(12):3615–27.
395. Latrofa F, Ricci D, Montanelli L, Rocchi R, Piaggi P, Sisti E, et al. Lymphocytic thyroiditis on histology correlates with serum thyroglobulin autoantibodies in patients with papillary thyroid carcinoma: impact on detection of serum thyroglobulin. J Clin Endocrinol Metab. 2012; 97(7):2380–7.

396. Giovanella L, Suriano S, Ceriani L, Verburg FA. Undetectable thyroglobulin in patients with differentiated thyroid carcinoma and residual radioiodine uptake on a postablation whole-body scan. Clin Nucl Med. 2011; 36(2):109–12.

397. Cherk MH, Francis P, Topliss DJ, Bailey M, Kalff V. Incidence and implications of negative serum thyroglobulin but positive I-131 whole-body scans in patients with welldifferentiated thyroid cancer prepared with rhTSH or thyroid hormone withdrawal. Clin Endocrinol (Oxf). 2012; 76(5):734–40.

398. Torlontano M, Crocetti U, Augello G, D'Aloiso L, Bonfitto N, Varraso A, et al. Comparative evaluation of recombinant human thyrotropin-stimulated thyroglobulin levels, 131I wholebody scintigraphy, and neck ultrasonography in the follow-up of patients with papillary thyroid microcarcinoma who have not undergone radioiodine therapy. J Clin Endocrinol Metab. 2006; 91(1):60–3.
399. Pacini F, Agate L, Elisei R, Capezzone M, Ceccarelli C, Lippi F, et al. Outcome of differentiated thyroid cancer with detectable serum Tg and negative diagnostic (131)I whole body scan: comparison of patients treated with high (131)I activities versus untreated patients. J Clin Endocrinol Metab. 2001; 86(9):4092–7.

400. Frasoldati A, Pesenti M, Gallo M, Caroggio A, Salvo D, Valcavi R. Diagnosis of neck recurrences in patients with differentiated thyroid carcinoma. Cancer. 2003; 97(1):90–6.

401. Diaz-Soto G, Puig-Domingo M, Martinez-Pino I, Martinez de Osaba MJ, Mora M, Rivera-Fillat F, et al. Do thyroid cancer patients with basal undetectable Tg measured by current immunoassays require rhTSH testing? Exp Clin Endocrinol Diabetes. 2011; 119(6):348–52.

402. Nascimento C, Borget I, Al Ghuzlan A, Deandreis D, Chami L, Travagli JP, et al. Persistent disease and recurrence in differentiated thyroid cancer patients with undetectable postoperative stimulated thyroglobulin level. Endocr Relat Cancer. 2011; 18(2):R29–40.

403. Mazzaferri EL, Robbins RJ, Spencer CA, Braverman LE, Pacini F, Wartofsky L, et al. A consensus report of the role of serum thyroglobulin as a monitoring method for low-risk patients with papillary thyroid carcinoma. J Clin Endocrinol Metab. 2003; 88(4):1433–41.

404. Iervasi A, Iervasi G, Ferdeghini M, Solimeo C, Bottoni A, Rossi L, et al. Clinical relevance of highly sensitive Tg assay in monitoring patients treated for differentiated thyroid cancer. Clin Endocrinol (Oxf). 2007; 67(3):434–41.

405. Padovani RP, Robenshtok E, Brokhin M, Tuttle RM. Even without additional therapy, serum thyroglobulin concentrations often decline for years after total thyroidectomy and radioactive remnant ablation in patients with differentiated thyroid cancer. Thyroid. 2012; 22(8):778–83.

406. Haugen BR, Pacini F, Reiners C, Schlumberger M, Ladenson PW, Sherman SI, et al. A comparison of recombinant human thyrotropin and thyroid hormone withdrawal for the detection of thyroid remnant or cancer. J Clin Endocrinol Metab. 1999; 84(11):3877–85.

407. David A, Blotta A, Bondanelli M, Rossi R, Roti E, Braverman LE, et al. Serum thyroglobulin concentrations and (131)I whole-body scan results in patients with differentiated thyroid carcinoma after administration of recombinant human thyroid-stimulating hormone. J Nucl Med. 2001; 42(10):1470–5.
408. Mazzaferri EL, Kloos RT. Is diagnostic iodine-131 scanning with recombinant human TSH useful in the follow-up of differentiated thyroid cancer after thyroid ablation? J Clin Endocrinol Metab. 2002; 87(4):1490–8.
409. Haugen BR, Ridgway EC, McLaughlin BA, McDermott MT. Clinical comparison of whole-body radioiodine scan and serum thyroglobulin after stimulation with recombinant human thyrotropin. Thyroid. 2002; 12(1):37–43.

410. Lima N, Cavaliere H, Tomimori E, Knobel M, Medeiros-Neto G. Prognostic value of serial serum thyroglobulin determinations after total thyroidectomy for differentiated thyroid cancer. J Endocrinol Invest. 2002; 25(2):110–5.

411. Wartofsky L, rhTSH-Stimulated Thyroglobulin Study Group. Management of low-risk well-differentiated thyroid cancer based only on thyroglobulin measurement after recombinant human thyrotropin. Thyroid. 2002; 12(7):583–90.
412. Pacini F, Sabra MM, Tuttle RM. Clinical relevance of thyroglobulin doubling time in the management of patients with differentiated thyroid cancer. Thyroid. 2011; 21(7):691–2.

413. Spencer C, Fatemi S. Thyroglobulin antibody (TgAb) methods – Strengths, pitfalls and clinical utility for monitoring TgAb-positive patients with differentiated thyroid cancer. Best Pract Res Clin Endocrinol Metab. 2013; 27(5):701–12.

414. Durante C, Montesano T, Attard M, Torlontano M, Monzani F, Costante G, et al. Long-term surveillance of papillary thyroid cancer patients who do not undergo postoperative radioiodine remnant ablation: is there a role for serum thyroglobulin measurement? J Clin Endocrinol Metab. 2012; 97(8):2748–53.

415. Pacini F, Molinaro E, Castagna MG, Agate L, Elisei R, Ceccarelli C, et al. Recombinant human thyrotropin-stimulated serum thyroglobulin combined with neck ultrasonography has the highest sensitivity in monitoring differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2003; 88(8):3668–73.

416. Shin JH, Han BK, Ko EY, Kang SS. Sonographic findings in the surgical bed after thyroidectomy: comparison of recurrent tumors and nonrecurrent lesions. J Ultrasound Med. 2007; 26(10):1359–66.
417. Bardet S, Malville E, Rame JP, Babin E, Samama G, De Raucourt D, et al. Macroscopic lymph-node involvement and neck dissection predict lymph-node recurrence in papillary thyroid carcinoma. Eur J Endocrinol. 2008; 158(4):551–60.

418. Spencer C, Fatemi S, Singer P, Nicoloff J, Lopresti J. Serum Basal thyroglobulin measured by a second-generation assay correlates with the recombinant human thyrotropin-stimulated thyroglobulin response in patients treated for differentiated thyroid cancer. Thyroid. 2010; 20(6):587–95.

419. Pacini F, Fugazzola L, Lippi F, Ceccarelli C, Centoni R, Miccoli P, et al. Detection of thyroglobulin in fine needle aspirates of nonthyroidal neck masses: a clue to the diagnosis of metastatic differentiated thyroid cancer. J Clin Endocrinol Metab. 1992; 74(6):1401–4.

420. Torres MR, Nobrega Neto SH, Rosas RJ, Martins AL, Ramos AL, da Cruz TR. Thyroglobulin in the washout fluid of lymph-node biopsy: what is its role in the follow-up of differentiated thyroid carcinoma? Thyroid. 2014; 24(1):7–18.

421. Frasoldati A, Toschi E, Zini M, Flora M, Caroggio A, Dotti C, et al. Role of thyroglobulin measurement in fine-needle aspiration biopsies of cervical lymph nodes in patients with differentiated thyroid cancer. Thyroid. 1999; 9(2):105–11.

422. Snozek CL, Chambers EP, Reading CC, Sebo TJ, Sistrunk JW, Singh RJ, et al. Serum thyroglobulin, high-resolution ultrasound, and lymph node thyroglobulin in diagnosis of differentiated thyroid carcinoma nodal metastases. J Clin Endocrinol Metab. 2007; 92(11):4278–81.

423. Boi F, Baghino G, Atzeni F, Lai ML, Faa G, Mariotti S. The diagnostic value for differentiated thyroid carcinoma metastases of thyroglobulin (Tg) measurement in washout fluid from fine-needle aspiration biopsy of neck lymph nodes is maintained in the presence of circulating anti-Tg antibodies. J Clin Endocrinol Metab. 2006; 91(4):1364–9.

424. Baloch ZW, Barroeta JE, Walsh J, Gupta PK, Livolsi VA, Langer JE, et al. Utility of Thyroglobulin measurement in fine-needle aspiration biopsy specimens of lymph nodes in the diagnosis of recurrent thyroid carcinoma. Cytojournal. 2008; 5:1.

425. Pacini F, Capezzone M, Elisei R, Ceccarelli C, Taddei D, Pinchera A. Diagnostic 131-iodine whole-body scan may be avoided in thyroid cancer patients who have undetectable stimulated serum Tg levels after initial treatment. J Clin Endocrinol Metab. 2002; 87(4):1499–501.

426. Torlontano M, Crocetti U, D'Aloiso L, Bonfitto N, Di Giorgio A, Modoni S, et al. Serum thyroglobulin and 131I whole body scan after recombinant human TSH stimulation in the follow-up of low-risk patients with differentiated thyroid cancer. Eur J Endocrinol. 2003; 148(1):19–24.

427. Aide N, Heutte N, Rame JP, Rousseau E, Loiseau C, Henry-Amar M, et al. Clinical relevance of single-photon emission computed tomography/computed tomography of the neck and thorax in postablation (131)I scintigraphy for thyroid cancer. J Clin Endocrinol Metab. 2009; 94(6):2075–84.

428. Schmidt D, Szikszai A, Linke R, Bautz W, Kuwert T. Impact of 131I SPECT/spiral CT on nodal staging of differentiated thyroid carcinoma at the first radioablation. J Nucl Med. 2009; 50(1):18–23.

429. Jeong SY, Lee SW, Kim HW, Song BI, Ahn BC, Lee J. Clinical applications of SPECT/CT after first I-131 ablation in patients with differentiated thyroid cancer. Clin Endocrinol (Oxf). 2014; 81(3):445–51.

430. Tharp K, Israel O, Hausmann J, Bettman L, Martin WH, Daitzchman M, et al. Impact of 131I-SPECT/CT images obtained with an integrated system in the follow-up of patients with thyroid carcinoma. Eur J Nucl Med Mol Imaging. 2004; 31(10):1435–42.

431. Leboulleux S, Schroeder PR, Schlumberger M, Ladenson PW. The role of PET in follow-up of patients treated for differentiated epithelial thyroid cancers. Nat Clin Pract Endocrinol Metab. 2007; 3(2):112–21.

432. Robbins RJ, Wan Q, Grewal RK, Reibke R, Gonen M, Strauss HW, et al. Realtime prognosis for metastatic thyroid carcinoma based on 2-[18F]fluoro-2-deoxy-D-glucose-positron emission tomography scanning. J Clin Endocrinol Metab. 2006; 91(2):498–505.

433. Deandreis D, Al Ghuzlan A, Leboulleux S, Lacroix L, Garsi JP, Talbot M, et al. Do histological, immunohistochemical, and metabolic (radioiodine and fluorodeoxyglucose uptakes) patterns of metastatic thyroid cancer correlate with patient outcome? Endocr Relat Cancer. 2011; 18(1):159–69.

434. Leboulleux S, Schroeder PR, Busaidy NL, Auperin A, Corone C, Jacene HA, et al. Assessment of the incremental value of recombinant thyrotropin stimulation before 2-[18F]-Fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography imaging to localize residual differentiated thyroid cancer. J Clin Endocrinol Metab. 2009; 94(4):1310–6.

435. Yoon DY, Hwang HS, Chang SK, Rho YS, Ahn HY, Kim JH, et al. CT, MR, US, 18F-FDG PET/CT, and their combined use for the assessment of cervical lymph node metastases in squamous cell carcinoma of the head and neck. Eur Radiol. 2009; 19(3):634–42.

436. Padovani RP, Kasamatsu TS, Nakabashi CC, Camacho CP, Andreoni DM, Malouf EZ, et al. One month is sufficient for urinary iodine to return to its baseline value after the use of water-soluble iodinated contrast agents in post-thyroidectomy patients requiring radioiodine therapy. Thyroid. 2012; 22(9):926–30.

437. Choi JS, Kim J, Kwak JY, Kim MJ, Chang HS, Kim EK. Preoperative staging of papillary thyroid carcinoma: comparison of ultrasound imaging and CT. AJR Am J Roentgenol. 2009; 193(3):871–8.

438. Takashima S, Sone S, Takayama F, Wang Q, Kobayashi T, Horii A, et al. Papillary thyroid carcinoma: MR diagnosis of lymph node metastasis. AJNR Am J Neuroradiol. 1998; 19(3):509–13.
439. Gross ND, Weissman JL, Talbot JM, Andersen PE, Wax MK, Cohen JI. MRI detection of cervical metastasis from differentiated thyroid carcinoma. Laryngoscope. 2001; 111(11 Pt 1):1905–9.

440. Toubert ME, Cyna-Gorse F, Zagdanski AM, Noel-Wekstein S, Cattan P, Billotey C, et al. Cervicomediastinal magnetic resonance imaging in persistent or recurrent papillary thyroid carcinoma: clinical use and limits. Thyroid. 1999; 9(6):591–7.

441. Wang JC, Takashima S, Takayama F, Kawakami S, Saito A, Matsushita T, et al. Tracheal invasion by thyroid carcinoma: prediction using MR imaging. AJR Am J Roentgenol. 2001; 177(4):929–36.
442. Wang J, Takashima S, Matsushita T, Takayama F, Kobayashi T, Kadoya M. Esophageal invasion by thyroid carcinomas: prediction using magnetic resonance imaging. J Comput Assist Tomogr. 2003; 27(1):18–25.

443. Lee DH, Kang WJ, Seo HS, Kim E, Kim JH, Son KR, et al. Detection of metastatic cervical lymph nodes in recurrent papillary thyroid carcinoma: computed tomography versus positron emission tomography-computed tomography. J Comput Assist Tomogr. 2009; 33(5):805–10.
444. Rosario PW, Mourao GF, dos Santos JB, Calsolari MR. Is empirical radioactive iodine therapy still a valid approach to patients with thyroid cancer and elevated thyroglobulin? Thyroid. 2014; 24(3):533–6.

445. Leboulleux S, El Bez I, Borget I, Elleuch M, Deandreis D, Al Ghuzlan A, et al. Postradioiodine treatment whole-body scan in the era of 18-fluorodeoxyglucose positron emission tomography for differentiated thyroid carcinoma with elevated serum thyroglobulin levels. Thyroid. 2012; 22(8):832–8.

446. Hovens GC, Stokkel MP, Kievit J, Corssmit EP, Pereira AM, Romijn JA, et al. Associations of serum thyrotropin concentrations with recurrence and death in differentiated thyroid cancer. J Clin Endocrinol Metab. 2007; 92(7):2610–5.

447. Sugitani I, Fujimoto Y. Does postoperative thyrotropin suppression therapy truly decrease recurrence in papillary thyroid carcinoma? A randomized controlled trial. J Clin Endocrinol Metab. 2010; 95(10):4576–83.

448. Klein Hesselink EN, Klein Hesselink MS, de Bock GH, Gansevoort RT, Bakker SJ, Vredeveld EJ, et al. Long-term cardiovascular mortality in patients with differentiated thyroid carcinoma: an observational study. J Clin Oncol. 2013; 31(32):4046–53.

449. Biondi B, Cooper DS. Benefits of thyrotropin suppression versus the risks of adverse effects in differentiated thyroid cancer. Thyroid. 2010; 20(2):135–46.

450. Shargorodsky M, Serov S, Gavish D, Leibovitz E, Harpaz D, Zimlichman R. Long-term thyrotropin-suppressive therapy with levothyroxine impairs small and large artery elasticity and increases left ventricular mass in patients with thyroid carcinoma. Thyroid. 2006; 16(4):381–6.

451. Taillard V, Sardinoux M, Oudot C, Fesler P, Rugale C, Raingeard I, et al. Early detection of isolated left ventricular diastolic dysfunction in high-risk differentiated thyroid carcinoma patients on TSH-suppressive therapy. Clin Endocrinol (Oxf). 2011; 75(5):709–14.

452. Dupuy DE, Monchik JM, Decrea C, Pisharodi L. Radiofrequency ablation of regional recurrence from welldifferentiated thyroid malignancy. Surgery. 2001; 130(6):971–7.

453. Lewis BD, Hay ID, Charboneau JW, McIver B, Reading CC, Goellner JR. Percutaneous ethanol injection for treatment of cervical lymph node metastases in patients with papillary thyroid carcinoma. AJR Am J Roentgenol. 2002; 178(3):699–704.

454. Eustatia-Rutten CF, Romijn JA, Guijt MJ, Vielvoye GJ, van den Berg R, Corssmit EP, et al. Outcome of palliative embolization of bone metastases in differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2003; 88(7):3184–9.

455. Tufano RP, Clayman G, Heller KS, Inabnet WB, Kebebew E, Shaha A, et al. Management of recurrent/persistent nodal disease in patients with differentiated thyroid cancer: a critical review of the risks and benefits of surgical intervention versus active surveillance. Thyroid. 2015; 25(1):15–27.

456. Phelan E, Kamani D, Shin J, Randolph GW. Neural monitored revision thyroid cancer surgery: surgical safety and thyroglobulin response. Otolaryngol Head Neck Surg. 2013; 149(1):47–52.
457. Urken ML, Milas M, Randolph GW, Tufano R, Bergman D, Bernet V, et al. Management of recurrent and persistent metastatic lymph nodes in well-differentiated thyroid cancer: a multifactorial decision-making guide for the Thyroid Cancer Care Collaborative. Head Neck. 2015; 37(4):605–14.

458. Merdad M, Eskander A, Kroeker T, Freeman JL. Predictors of level II and Vb neck disease in metastatic papillary thyroid cancer. Arch Otolaryngol Head Neck Surg. 2012; 138(11):1030–3.

459. Eskander A, Merdad M, Freeman JL, Witterick IJ. Pattern of spread to the lateral neck in metastatic well-differentiated thyroid cancer: a systematic review and metaanalysis. Thyroid. 2013; 23(5):583–92.

460. Schuff KG. Management of recurrent/persistent papillary thyroid carcinoma: efficacy of the surgical option. J Clin Endocrinol Metab. 2011; 96(7):2038–9.

461. McCoy KL, Yim JH, Tublin ME, Burmeister LA, Ogilvie JB, Carty SE. Same-day ultrasound guidance in reoperation for locally recurrent papillary thyroid cancer. Surgery. 2007; 142(6):965–72.

462. Hughes DT, Laird AM, Miller BS, Gauger PG, Doherty GM. Reoperative lymph node dissection for recurrent papillary thyroid cancer and effect on serum thyroglobulin. Ann Surg Oncol. 2012; 19(9):2951–7.

463. Roh JL, Kim JM, Park CI. Central compartment reoperation for recurrent/persistent differentiated thyroid cancer: patterns of recurrence, morbidity, and prediction of postoperative hypocalcemia. Ann Surg Oncol. 2011; 18(5):1312–8.

465. Palme CE, Waseem Z, Raza SN, Eski S, Walfish P, Freeman JL. Management and outcome of recurrent welldifferentiated thyroid carcinoma. Arch Otolaryngol Head Neck Surg. 2004; 130(7):819–24.

466. Clayman GL, Agarwal G, Edeiken BS, Waguespack SG, Roberts DB, Sherman SI. Long-term outcome of comprehensive central compartment dissection in patients with recurrent/persistent papillary thyroid carcinoma. Thyroid. 2011; 21(12):1309–16.

467. Clayman GL, Shellenberger TD, Ginsberg LE, Edeiken BS, El-Naggar AK, Sellin RV, et al. Approach and safety of comprehensive central compartment dissection in patients with recurrent papillary thyroid carcinoma. Head Neck. 2009; 31(9):1152–63.

468. Chao TC, Jeng LB, Lin JD, Chen MF. Reoperative thyroid surgery. World J Surg. 1997; 21(6):644–7.

469. Erbil Y, Sari S, Agcaoglu O, Ersoz F, Bayraktar A, Salmaslioglu A, et al. Radio-guided excision of metastatic lymph nodes in thyroid carcinoma: a safe technique for previously operated neck compartments. World J Surg. 2010; 34(11):2581–8.

470. Alzahrani AS, Raef H, Sultan A, Al Sobhi S, Ingemansson S, Ahmed M, et al. Impact of cervical lymph node dissection on serum TG and the course of disease in TG-positive, radioactive iodine whole body scan-negative recurrent/persistent papillary thyroid cancer. J Endocrinol Invest. 2002; 25(6):526–31.

471. Travagli JP, Cailleux AF, Ricard M, Baudin E, Caillou B, Parmentier C, et al. Combination of radioiodine (131I) and probe-guided surgery for persistent or recurrent thyroid carcinoma. J Clin Endocrinol Metab. 1998; 83(8):2675–80.

472. Lee L, Steward DL. Sonographically-directed neck dissection for recurrent thyroid carcinoma. Laryngoscope. 2008; 118(6):991–4.

473. Steward DL. Update in utility of secondary node dissection for papillary thyroid cancer. J Clin Endocrinol Metab. 2012; 97(10):3393–8.

474. Rubello D, Salvatori M, Casara D, Piotto A, Toniato A, Gross MD, et al. 99mTc-sestamibi radio-guided surgery of locoregional 131Iodine-negative recurrent thyroid cancer. Eur J Surg Oncol. 2007; 33(7):902–6.

475. Shin JE, Baek JH, Lee JH. Radiofrequency and ethanol ablation for the treatment of recurrent thyroid cancers: current status and challenges. Curr Opin Oncol. 2013; 25(1):14–9.
476. Baek JH, Kim YS, Sung JY, Choi H, Lee JH. Locoregional control of metastatic well-differentiated thyroid cancer by ultrasound-guided radiofrequency ablation. AJR Am J Roentgenol. 2011; 197(2):W331–6.

477. Perros P, Boelaert K, Colley S, Evans C, Evans RM, Gerrard Ba G, et al. Guidelines for the management of thyroid cancer. Clin Endocrinol (Oxf). 2014; 81(Suppl 1):1–122.

478. Kim JH, Yoo WS, Park YJ, Park DJ, Yun TJ, Choi SH, et al. Efficacy and safety of radiofrequency ablation for treatment of locally recurrent thyroid cancers smaller than 2 cm. Radiology. 2015; 276(3):909–18.

479. Suh CH, Baek JH, Choi YJ, Lee JH. Efficacy and safety of radiofrequency and ethanol ablation for treating locally recurrent thyroid cancer: a systematic review and metaanalysis. Thyroid. 2016; 26(3):420–8.

480. Owen RP, Silver CE, Ravikumar TS, Brook A, Bello J, Breining D. Techniques for radiofrequency ablation of head and neck tumors. Arch Otolaryngol Head Neck Surg. 2004; 130(1):52–6.

481. Ge JH, Zhao RL, Hu JL, Zhou WA. Surgical treatment of advanced thyroid carcinoma with aerodigestive invasion. Zhonghua Er Bi Yan Hou Ke Za Zhi. 2004; 39(4):237–40.
482. Avenia N, Ragusa M, Monacelli M, Calzolari F, Daddi N, Di Carlo L, et al. Locally advanced thyroid cancer: therapeutic options. Chir Ital. 2004; 56(4):501–8.
483. McCaffrey JC. Evaluation and treatment of aerodigestive tract invasion by well-differentiated thyroid carcinoma. Cancer Control. 2000; 7(3):246–52.

484. Musholt TJ, Musholt PB, Behrend M, Raab R, Scheumann GF, Klempnauer J. Invasive differentiated thyroid carcinoma: tracheal resection and reconstruction procedures in the hands of the endocrine surgeon. Surgery. 1999; 126(6):1078–87. ; discussion 87–8.

485. Czaja JM, McCaffrey TV. The surgical management of laryngotracheal invasion by well-differentiated papillary thyroid carcinoma. Arch Otolaryngol Head Neck Surg. 1997; 123(5):484–90.

486. Shindo ML, Caruana SM, Kandil E, McCaffrey JC, Orloff LA, Porterfield JR, et al. Management of invasive well-differentiated thyroid cancer: an American Head and Neck Society consensus statement. AHNS consensus statement. Head Neck. 2014; 36(10):1379–90.

487. Haymart MR, Muenz DG, Stewart AK, Griggs JJ, Banerjee M. Disease severity and radioactive iodine use for thyroid cancer. J Clin Endocrinol Metab. 2013; 98(2):678–86.

488. Van Nostrand D. The benefits and risks of I-131 therapy in patients with well-differentiated thyroid cancer. Thyroid. 2009; 19(12):1381–91.

489. Van Nostrand D, Wartofsky L. Radioiodine in the treatment of thyroid cancer. Endocrinol Metab Clin North Am. 2007; 36(3):807–22. vii-viii.

490. Van Nostrand D, Atkins F, Yeganeh F, Acio E, Bursaw R, Wartofsky L. Dosimetrically determined doses of radioiodine for the treatment of metastatic thyroid carcinoma. Thyroid. 2002; 12(2):121–34.

491. Chiesa C, Castellani MR, Vellani C, Orunesu E, Negri A, Azzeroni R, et al. Individualized dosimetry in the management of metastatic differentiated thyroid cancer. Q J Nucl Med Mol Imaging. 2009; 53(5):546–61.
492. Thomas SR, Maxon HR, Kereiakes JG. In vivo quantitation of lesion radioactivity using external counting methods. Med Phys. 1976; 03(04):253–5.

493. Holst JP, Burman KD, Atkins F, Umans JG, Jonklaas J. Radioiodine therapy for thyroid cancer and hyperthyroidism in patients with end-stage renal disease on hemodialysis. Thyroid. 2005; 15(12):1321–31.

494. Driedger AA, Quirk S, McDonald TJ, Ledger S, Gray D, Wall W, et al. A pragmatic protocol for I-131 rhTSH-stimulated ablation therapy in patients with renal failure. Clin Nucl Med. 2006; 31(8):454–7.

495. Jarzab B, Handkiewicz-Junak D, Wloch J. Juvenile differentiated thyroid carcinoma and the role of radioiodine in its treatment: a qualitative review. Endocr Relat Cancer. 2005; 12(4):773–803.

496. Verburg FA, Biko J, Diessl S, Demidchik Y, Drozd V, Rivkees SA, et al. I-131 activities as high as safely administrable (AHASA) for the treatment of children and adolescents with advanced differentiated thyroid cancer. J Clin Endocrinol Metab. 2011; 96(8):E1268–71.

497. Ma C, Xie J, Liu W, Wang G, Zuo S, Wang X, et al. Recombinant human thyrotropin (rhTSH) aided radioiodine treatment for residual or metastatic differentiated thyroid cancer. Cochrane Database Syst Rev. 2010(11):): CD008302.

498. Sgouros G, Kolbert KS, Sheikh A, Pentlow KS, Mun EF, Barth A, et al. Patientspecific dosimetry for 131I thyroid cancer therapy using 124I PET and 3-dimensional-internal dosimetry (3D-ID) software. J Nucl Med. 2004; 45(8):1366–72.
499. Jentzen W, Freudenberg L, Eising EG, Sonnenschein W, Knust J, Bockisch A. Optimized 124I PET dosimetry protocol for radioiodine therapy of differentiated thyroid cancer. J Nucl Med. 2008; 49(6):1017–23.

500. Pettinato C, Monari F, Nanni C, Allegri V, Marcatili S, Civollani S, et al. Usefulness of 124I PET/CT imaging to predict absorbed doses in patients affected by metastatic thyroid cancer and treated with 131I. Q J Nucl Med Mol Imaging. 2012; 56(6):509–14.
501. Schlumberger M, Lacroix L, Russo D, Filetti S, Bidart JM. Defects in iodide metabolism in thyroid cancer and implications for the follow-up and treatment of patients. Nat Clin Pract Endocrinol Metab. 2007; 3(3):260–9.

502. Kulkarni K, Van Nostrand D, Atkins F, Aiken M, Burman K, Wartofsky L. The relative frequency in which empiric dosages of radioiodine would potentially overtreat or undertreat patients who have metastatic well-differentiated thyroid cancer. Thyroid. 2006; 16(10):1019–23.

503. Tuttle RM, Leboeuf R, Robbins RJ, Qualey R, Pentlow K, Larson SM, et al. Empiric radioactive iodine dosing regimens frequently exceed maximum tolerated activity levels in elderly patients with thyroid cancer. J Nucl Med. 2006; 47(10):1587–91.
504. Klubo-Gwiezdzinska J, Burman KD, Van Nostrand D, Mete M, Jonklaas J, Wartofsky L. Radioiodine treatment of metastatic thyroid cancer: relative efficacy and side effect profile of preparation by thyroid hormone withdrawal versus recombinant human thyrotropin. Thyroid. 2012; 22(3):310–7.

505. Rudavsky AZ, Freeman LM. Treatment of scan-negative, thyroglobulin-positive metastatic thyroid cancer using radioiodine 131I and recombinant human thyroid stimulating hormone. J Clin Endocrinol Metab. 1997; 82(1):11–4.
506. Ringel MD, Ladenson PW. Diagnostic accuracy of 131I scanning with recombinant human thyrotropin versus thyroid hormone withdrawal in a patient with metastatic thyroid carcinoma and hypopituitarism. J Clin Endocrinol Metab. 1996; 81(5):1724–5.

507. Luster M, Lassmann M, Haenscheid H, Michalowski U, Incerti C, Reiners C. Use of recombinant human thyrotropin before radioiodine therapy in patients with advanced differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2000; 85(10):3640–5.

508. Mariani G, Ferdeghini M, Augeri C, Villa G, Taddei GZ, Scopinaro G, et al. Clinical experience with recombinant human thyrotrophin (rhTSH) in the management of patients with differentiated thyroid cancer. Cancer Biother Radiopharm. 2000; 15(2):211–7.

509. Perros P. Recombinant human thyroid-stimulating hormone (rhTSH) in the radioablation of well-differentiated thyroid cancer: preliminary therapeutic experience. J Endocrinol Invest. 1999; 22(11 Suppl):30–4.
510. Lippi F, Capezzone M, Angelini F, Taddei D, Molinaro E, Pinchera A, et al. Radioiodine treatment of metastatic differentiated thyroid cancer in patients on L-thyroxine, using recombinant human TSH. Eur J Endocrinol. 2001; 144(1):5–11.

511. Pellegriti G, Scollo C, Giuffrida D, Vigneri R, Squatrito S, Pezzino V. Usefulness of recombinant human thyrotropin in the radiometabolic treatment of selected patients with thyroid cancer. Thyroid. 2001; 11(11):1025–30.

512. Adler ML, Macapinlac HA, Robbins RJ. Radioiodine treatment of thyroid cancer with the aid of recombinant human thyrotropin. Endocr Pract. 1998; 4(5):282–6.

513. Chiu AC, Delpassand ES, Sherman SI. Prognosis and treatment of brain metastases in thyroid carcinoma. J Clin Endocrinol Metab. 1997; 82(11):3637–42.

514. Lau WF, Zacharin MR, Waters K, Wheeler G, Johnston V, Hicks RJ. Management of paediatric thyroid carcinoma: recent experience with recombinant human thyroid stimulating hormone in preparation for radioiodine therapy. Intern Med J. 2006; 36(9):564–70.

515. Potzi C, Moameni A, Karanikas G, Preitfellner J, Becherer A, Pirich C, et al. Comparison of iodine uptake in tumour and nontumour tissue under thyroid hormone deprivation and with recombinant human thyrotropin in thyroid cancer patients. Clin Endocrinol (Oxf). 2006; 65(4):519–23.
516. Pons F, Carrio I, Estorch M, Ginjaume M, Pons J, Milian R. Lithium as an adjuvant of iodine-131 uptake when treating patients with well-differentiated thyroid carcinoma. Clin Nucl Med. 1987; 12(8):644–7.
517. Koong SS, Reynolds JC, Movius EG, Keenan AM, Ain KB, Lakshmanan MC, et al. Lithium as a potential adjuvant to 131I therapy of metastatic, well differentiated thyroid carcinoma. J Clin Endocrinol Metab. 1999; 84(3):912–6.

518. Liu YY, van der Pluijm G, Karperien M, Stokkel MP, Pereira AM, Morreau J, et al. Lithium as adjuvant to radioiodine therapy in differentiated thyroid carcinoma: clinical and in vitro studies. Clin Endocrinol (Oxf). 2006; 64(6):617–24.

519. Lin JD, Chao TC, Chou SC, Hsueh C. Papillary thyroid carcinomas with lung metastases. Thyroid. 2004; 14(12):1091–6.

520. Shoup M, Stojadinovic A, Nissan A, Ghossein RA, Freedman S, Brennan MF, et al. Prognostic indicators of outcomes in patients with distant metastases from differentiated thyroid carcinoma. J Am Coll Surg. 2003; 197(2):191–7.

521. Pittas AG, Adler M, Fazzari M, Tickoo S, Rosai J, Larson SM, et al. Bone metastases from thyroid carcinoma: clinical characteristics and prognostic variables in one hundred forty-six patients. Thyroid. 2000; 10(3):261–8.

522. Dinneen SF, Valimaki MJ, Bergstralh EJ, Goellner JR, Gorman CA, Hay ID. Distant metastases in papillary thyroid carcinoma: 100 cases observed at one institution during 5 decades. J Clin Endocrinol Metab. 1995; 80(7):2041–5.

523. Foote RL, Brown PD, Garces YI, McIver B, Kasperbauer JL. Is there a role for radiation therapy in the management of Hurthle cell carcinoma? Int J Radiat Oncol Biol Phys. 2003; 56(4):1067–72.
524. Pak H, Gourgiotis L, Chang WI, Guthrie LC, Skarulis MC, Reynolds JC, et al. Role of metastasectomy in the management of thyroid carcinoma: the NIH experience. J Surg Oncol. 2003; 82(1):10–8.

525. Vitale G, Fonderico F, Martignetti A, Caraglia M, Ciccarelli A, Nuzzo V, et al. Pamidronate improves the quality of life and induces clinical remission of bone metastases in patients with thyroid cancer. Br J Cancer. 2001; 84(12):1586–90.

526. Kitamura Y, Shimizu K, Nagahama M, Sugino K, Ozaki O, Mimura T, et al. Immediate causes of death in thyroid carcinoma: clinicopathological analysis of 161 fatal cases. J Clin Endocrinol Metab. 1999; 84(11):4043–9.

527. Brose MS, Smit J, Capdevila J, Elisei R, Nutting C, Pitoia F, et al. Regional approaches to the management of patients with advanced, radioactive iodine-refractory differentiated thyroid carcinoma. Expert Rev Anticancer Ther. 2012; 12(9):1137–47.

528. Sabra MM, Dominguez JM, Grewal RK, Larson SM, Ghossein RA, Tuttle RM, et al. Clinical outcomes and molecular profile of differentiated thyroid cancers with radioiodine-avid distant metastases. J Clin Endocrinol Metab. 2013; 98(5):E829–36.

529. Ho AL, Grewal RK, Leboeuf R, Sherman EJ, Pfister DG, Deandreis D, et al. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N Engl J Med. 2013; 368(7):623–32.

530. Benua RS, Cicale NR, Sonenberg M, Rawson RW. The relation of radioiodine dosimetry to results and complications in the treatment of metastatic thyroid cancer. Am J Roentgenol Radium Ther Nucl Med. 1962; 87:171–82.
531. Hod N, Hagag P, Baumer M, Sandbank J, Horne T. Differentiated thyroid carcinoma in children and young adults: evaluation of response to treatment. Clin Nucl Med. 2005; 30(6):387–90.

532. Fatourechi V, Hay ID, Javedan H, Wiseman GA, Mullan BP, Gorman CA. Lack of impact of radioiodine therapy in tg-positive, diagnostic whole-body scan-negative patients with follicular cell-derived thyroid cancer. J Clin Endocrinol Metab. 2002; 87(4):1521–6.

533. Koh JM, Kim ES, Ryu JS, Hong SJ, Kim WB, Shong YK. Effects of therapeutic doses of 131I in thyroid papillary carcinoma patients with elevated thyroglobulin level and negative 131I whole-body scan: comparative study. Clin Endocrinol (Oxf). 2003; 58(4):421–7.

534. Ma C, Kuang A, Xie J. Radioiodine therapy for differentiated thyroid carcinoma with thyroglobulin positive and radioactive iodine negative metastases. Cochrane Database Syst Rev. 2009(1):): CD006988.

535. Sabra MM, Grewal RK, Tala H, Larson SM, Tuttle RM. Clinical outcomes following empiric radioiodine therapy in patients with structurally identifiable metastatic follicular cell-derived thyroid carcinoma with negative diagnostic but positive posttherapy 131I whole-body scans. Thyroid. 2012; 22(9):877–83.

536. Wang W, Larson SM, Tuttle RM, Kalaigian H, Kolbert K, Sonenberg M, et al. Resistance of [18f]-fluorodeoxyglucose-avid metastatic thyroid cancer lesions to treatment with high-dose radioactive iodine. Thyroid. 2001; 11(12):1169–75.

537. Salvatore B, Paone G, Klain M, Storto G, Nicolai E, D'Amico D, et al. Fluorodeoxyglucose PET/CT in patients with differentiated thyroid cancer and elevated thyroglobulin after total thyroidectomy and (131)I ablation. Q J Nucl Med Mol Imaging. 2008; 52(1):2–8.
538. Kloos RT. Approach to the patient with a positive serum thyroglobulin and a negative radioiodine scan after initial therapy for differentiated thyroid cancer. J Clin Endocrinol Metab. 2008; 93(5):1519–25.

539. van Tol KM, Jager PL, de Vries EG, Piers DA, Boezen HM, Sluiter WJ, et al. Outcome in patients with differentiated thyroid cancer with negative diagnostic whole-body scanning and detectable stimulated thyroglobulin. Eur J Endocrinol. 2003; 148(6):589–96.

540. Kabasakal L, Selcuk NA, Shafipour H, Ozmen O, Onsel C, Uslu I. Treatment of iodine-negative thyroglobulin-positive thyroid cancer: differences in outcome in patients with macrometastases and patients with micrometastases. Eur J Nucl Med Mol Imaging. 2004; 31(11):1500–4.

541. Yim JH, Kim EY, Bae Kim W, Kim WG, Kim TY, Ryu JS, et al. Long-term consequence of elevated thyroglobulin in differentiated thyroid cancer. Thyroid. 2013; 23(1):58–63.

542. Black EG, Sheppard MC, Hoffenberg R. Serial serum thyroglobulin measurements in the management of differentiated thyroid carcinoma. Clin Endocrinol (Oxf). 1987; 27(1):115–20.

543. Huang SH, Wang PW, Huang YE, Chou FF, Liu RT, Tung SC, et al. Sequential follow-up of serum thyroglobulin and whole body scan in thyroid cancer patients without initial metastasis. Thyroid. 2006; 16(12):1273–8.

544. Schlumberger M, Mancusi F, Baudin E, Pacini F. 131I therapy for elevated thyroglobulin levels. Thyroid. 1997; 7(2):273–6.

545. Ma C, Xie J, Kuang A. Is empiric 131I therapy justified for patients with positive thyroglobulin and negative 131I whole-body scanning results? J Nucl Med. 2005; 46(7):1164–70.
546. Chao M. Management of differentiated thyroid cancer with rising thyroglobulin and negative diagnostic radioiodine whole body scan. Clin Oncol (R Coll Radiol). 2010; 22(6):438–47.

547. Sinha P, Conrad GR, West HC. Response of thyroglobulin to radioiodine therapy in thyroglobulin-elevated negative iodine scintigraphy (TENIS) syndrome. Anticancer Res. 2011; 31(6):2109–12.
548. Ozata M, Suzuki S, Miyamoto T, Liu RT, Fierro-Renoy F, DeGroot LJ. Serum thyroglobulin in the follow-up of patients with treated differentiated thyroid cancer. J Clin Endocrinol Metab. 1994; 79(1):98–105.

549. Kim WG, Ryu JS, Kim EY, Lee JH, Baek JH, Yoon JH, et al. Empiric high-dose 131-iodine therapy lacks efficacy for treated papillary thyroid cancer patients with detectable serum thyroglobulin, but negative cervical sonography and 18Ffluorodeoxyglucose positron emission tomography scan. J Clin Endocrinol Metab. 2010; 95(3):1169–73.

550. Biko J, Reiners C, Kreissl MC, Verburg FA, Demidchik Y, Drozd V. Favourable course of disease after incomplete remission on (131)I therapy in children with pulmonary metastases of papillary thyroid carcinoma: 10 years follow-up. Eur J Nucl Med Mol Imaging. 2011; 38(4):651–5.
551. Walter MA, Turtschi CP, Schindler C, Minnig P, Muller-Brand J, Muller B. The dental safety profile of high-dose radioiodine therapy for thyroid cancer: longterm results of a longitudinal cohort study. J Nucl Med. 2007; 48(10):1620–5.

552. Kloos RT, Duvuuri V, Jhiang SM, Cahill KV, Foster JA, Burns JA. Nasolacrimal drainage system obstruction from radioactive iodine therapy for thyroid carcinoma. J Clin Endocrinol Metab. 2002; 87(12):5817–20.

553. Brown AP, Chen J, Hitchcock YJ, Szabo A, Shrieve DC, Tward JD. The risk of second primary malignancies up to three decades after the treatment of differentiated thyroid cancer. J Clin Endocrinol Metab. 2008; 93(2):504–15.

554. Rubino C, de Vathaire F, Dottorini ME, Hall P, Schvartz C, Couette JE, et al. Second primary malignancies in thyroid cancer patients. Br J Cancer. 2003; 89(9):1638–44.

555. Sandeep TC, Strachan MW, Reynolds RM, Brewster DH, Scelo G, Pukkala E, et al. Second primary cancers in thyroid cancer patients: a multinational record linkage study. J Clin Endocrinol Metab. 2006; 91(5):1819–25.

556. Subramanian S, Goldstein DP, Parlea L, Thabane L, Ezzat S, Ibrahim-Zada I, et al. Second primary malignancy risk in thyroid cancer survivors: a systematic review and metaanalysis. Thyroid. 2007; 17(12):1277–88.

557. Nakada K, Ishibashi T, Takei T, Hirata K, Shinohara K, Katoh S, et al. Does lemon candy decrease salivary gland damage after radioiodine therapy for thyroid cancer? J Nucl Med. 2005; 46(2):261–6.
558. Jentzen W, Balschuweit D, Schmitz J, Freudenberg L, Eising E, Hilbel T, et al. The influence of saliva flow stimulation on the absorbed radiation dose to the salivary glands during radioiodine therapy of thyroid cancer using 124I PET(/CT) imaging. Eur J Nucl Med Mol Imaging. 2010; 37(12):2298–306.

559. Van Nostrand D, Bandaru V, Chennupati S, Wexler J, Kulkarni K, Atkins F, et al. Radiopharmacokinetics of radioiodine in the parotid glands after the administration of lemon juice. Thyroid. 2010; 20(10):1113–9.

561. Bomeli SR, Schaitkin B, Carrau RL, Walvekar RR. Interventional sialendoscopy for treatment of radioiodineinduced sialadenitis. Laryngoscope. 2009; 119(5):864–7.

562. Prendes BL, Orloff LA, Eisele DW. Therapeutic sialendoscopy for the management of radioiodine sialadenitis. Arch Otolaryngol Head Neck Surg. 2012; 138(1):15–9.

563. Bhayani MK, Acharya V, Kongkiatkamon S, Farah S, Roberts DB, Sterba J, et al. Sialendoscopy for Patients with Radioiodine-Induced Sialadenitis and Xerostomia. Thyroid. 2015; 25(7):834–8.

564. Sawka AM, Thabane L, Parlea L, Ibrahim-Zada I, Tsang RW, Brierley JD, et al. Second primary malignancy risk after radioactive iodine treatment for thyroid cancer: a systematic review and metaanalysis. Thyroid. 2009; 19(5):451–7.

565. Iyer NG, Morris LG, Tuttle RM, Shaha AR, Ganly I. Rising incidence of second cancers in patients with low-risk (T1N0) thyroid cancer who receive radioactive iodine therapy. Cancer. 2011; 117(19):4439–46.

566. Chen AY, Levy L, Goepfert H, Brown BW, Spitz MR, Vassilopoulou-Sellin R. The development of breast carcinoma in women with thyroid carcinoma. Cancer. 2001; 92(2):225–31.

567. Berthe E, Henry-Amar M, Michels JJ, Rame JP, Berthet P, Babin E, et al. Risk of second primary cancer following differentiated thyroid cancer. Eur J Nucl Med Mol Imaging. 2004; 31(5):685–91.

568. Luster M, Clarke SE, Dietlein M, Lassmann M, Lind P, Oyen WJ, et al. Guidelines for radioiodine therapy of differentiated thyroid cancer. Eur J Nucl Med Mol Imaging. 2008; 35(10):1941–59.

569. Perry WF, Hughes JF. The urinary excretion and thyroid uptake of iodine in renal disease. J Clin Invest. 1952; 31(5):457–63.
570. Vini L, Hyer S, Al-Saadi A, Pratt B, Harmer C. Prognosis for fertility and ovarian function after treatment with radioiodine for thyroid cancer. Postgrad Med J. 2002; 78(916):92–3.

571. Dottorini ME, Lomuscio G, Mazzucchelli L, Vignati A, Colombo L. Assessment of female fertility and carcinogenesis after iodine-131 therapy for differentiated thyroid carcinoma. J Nucl Med. 1995; 36(1):21–7.
572. Sawka AM, Lakra DC, Lea J, Alshehri B, Tsang RW, Brierley JD, et al. A systematic review examining the effects of therapeutic radioactive iodine on ovarian function and future pregnancy in female thyroid cancer survivors. Clin Endocrinol (Oxf). 2008; 69(3):479–90.

573. Wu JX, Young S, Ro K, Li N, Leung AM, Chiu HK, et al. Reproductive outcomes and nononcologic complications after radioactive iodine ablation for well-differentiated thyroid cancer. Thyroid. 2015; 25(1):133–8.

574. American Thyroid Association Taskforce On Radioiodine Safety. Sisson JC, Freitas J, McDougall IR, Dauer LT, Hurley JR, et al. Radiation safety in the treatment of patients with thyroid diseases by radioiodine 131I: practice recommendations of the American Thyroid Association. Thyroid. 2011; 21(4):335–46.

575. Bernard N, Jantzem H, Becker M, Pecriaux C, Benard-Laribiere A, Montastruc JL, et al. Severe adverse effects of bromocriptine in lactation inhibition: a pharmacovigilance survey. BJOG. 2015; 122(9):1244–51.

576. Wichers M, Benz E, Palmedo H, Biersack HJ, Grunwald F, Klingmuller D. Testicular function after radioiodine therapy for thyroid carcinoma. Eur J Nucl Med. 2000; 27(5):503–7.

577. Hyer S, Vini L, O'Connell M, Pratt B, Harmer C. Testicular dose and fertility in men following I(131) therapy for thyroid cancer. Clin Endocrinol (Oxf). 2002; 56(6):755–8.

578. Lushbaugh CC, Casarett GW. The effects of gonadal irradiation in clinical radiation therapy: a review. Cancer. 1976; 37(2 Suppl):1111–25.

579. Sarkar SD, Beierwaltes WH, Gill SP, Cowley BJ. Subsequent fertility and birth histories of children and adolescents treated with 131I for thyroid cancer. J Nucl Med. 1976; 17(6):460–4.
580. Mazzaferri EL. Gonadal damage from 131I therapy for thyroid cancer. Clin Endocrinol (Oxf). 2002; 57(3):313–4.

581. Schlumberger M, Brose M, Elisei R, Leboulleux S, Luster M, Pitoia F, et al. Definition and management of radioactive iodine-refractory differentiated thyroid cancer. Lancet Diabetes Endocrinol. 2014; 2(5):356–8.

582. Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006; 91(8):2892–9.

583. Lo SS, Fakiris AJ, Chang EL, Mayr NA, Wang JZ, Papiez L, et al. Stereotactic body radiation therapy: a novel treatment modality. Nat Rev Clin Oncol. 2010; 7(1):44–54.

584. Lo SS, Fakiris AJ, Teh BS, Cardenes HR, Henderson MA, Forquer JA, et al. Stereotactic body radiation therapy for oligometastases. Expert Rev Anticancer Ther. 2009; 9(5):621–35.

585. Goetz MP, Callstrom MR, Charboneau JW, Farrell MA, Maus TP, Welch TJ, et al. Percutaneous imageguided radiofrequency ablation of painful metastases involving bone: a multicenter study. J Clin Oncol. 2004; 22(2):300–6.

586. Dupuy DE, Liu D, Hartfeil D, Hanna L, Blume JD, Ahrar K, et al. Percutaneous radiofrequency ablation of painful osseous metastases: a multicenter American College of Radiology Imaging Network trial. Cancer. 2010; 116(4):989–97.
587. Van Tol KM, Hew JM, Jager PL, Vermey A, Dullaart RP, Links TP. Embolization in combination with radioiodine therapy for bone metastases from differentiated thyroid carcinoma. Clin Endocrinol (Oxf). 2000; 52(5):653–9.

588. Coleman R, Woodward E, Brown J, Cameron D, Bell R, Dodwell D, et al. Safety of zoledronic acid and incidence of osteonecrosis of the jaw (ONJ) during adjuvant therapy in a randomised phase III trial (AZURE: BIG 01–04) for women with stage II/III breast cancer. Breast Cancer Res Treat. 2011; 127(2):429–38.

589. Wardley A, Davidson N, Barrett-Lee P, Hong A, Mansi J, Dodwell D, et al. Zoledronic acid significantly improves pain scores and quality of life in breast cancer patients with bone metastases: a randomised, crossover study of community vs hospital bisphosphonate administration. Br J Cancer. 2005; 92(10):1869–76.

590. Orita Y, Sugitani I, Toda K, Manabe J, Fujimoto Y. Zoledronic acid in the treatment of bone metastases from differentiated thyroid carcinoma. Thyroid. 2011; 21(1):31–5.

591. McWilliams RR, Giannini C, Hay ID, Atkinson JL, Stafford SL, Buckner JC. Management of brain metastases from thyroid carcinoma: a study of 16 pathologically confirmed cases over 25 years. Cancer. 2003; 98(2):356–62.
592. Anderson RT, Linnehan JE, Tongbram V, Keating K, Wirth LJ. Clinical, safety, and economic evidence in radioactive iodine-refractory differentiated thyroid cancer: a systematic literature review. Thyroid. 2013; 23(4):392–407.

593. Leboulleux S, Bastholt L, Krause T, de la Fouchardiere C, Tennvall J, Awada A, et al. Vandetanib in locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 2 trial. Lancet Oncol. 2012; 13(9):897–905.

594. Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. 2014; 384(9940):319–28.

595. Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 2015; 372(7):621–30.

596. Droz JP, Schlumberger M, Rougier P, Ghosn M, Gardet P, Parmentier C. Chemotherapy in metastatic nonanaplastic thyroid cancer: experience at the Institut Gustave-Roussy. Tumori. 1990; 76(5):480–3.

597. Schutz FA, Je Y, Richards CJ, Choueiri TK. Meta-analysis of randomized controlled trials for the incidence and risk of treatment-related mortality in patients with cancer treated with vascular endothelial growth factor tyrosine kinase inhibitors. J Clin Oncol. 2012; 30(8):871–7.

598. Cohen EE, Rosen LS, Vokes EE, Kies MS, Forastiere AA, Worden FP, et al. Axitinib is an active treatment for all histologic subtypes of advanced thyroid cancer: results from a phase II study. J Clin Oncol. 2008; 26(29):4708–13.

599. Bible KC, Suman VJ, Molina JR, Smallridge RC, Maples WJ, Menefee ME, et al. Efficacy of pazopanib in progressive, radioiodine-refractory, metastatic differentiated thyroid cancers: results of a phase 2 consortium study. Lancet Oncol. 2010; 11(10):962–72.

600. Carr LL, Mankoff DA, Goulart BH, Eaton KD, Capell PT, Kell EM, et al. Phase II study of daily sunitinib in FDG-PET-positive, iodine-refractory differentiated thyroid cancer and metastatic medullary carcinoma of the thyroid with functional imaging correlation. Clin Cancer Res. 2010; 16(21):5260–8.

Go to : 
Table 1.
갑상선결절 및 암 진료 권고안의 권고수준
Table 2.
K-TIRADS에 기초한 갑상선결절의 암 위험도 및 세침흡인검사 기준 a
| 카테고리 | 초음파 유형 | 암 위험도(%) | 계산된 암 위험도(%) | 세침흡인검사 c |
|---|---|---|---|---|
| 5 높은의심 | 암 의심 초음파 소견 b이 있는 저에코 고형결절 | >60 | 79 (61–85) | >1 cm (선택적으로 >0.5 cm d) |
| 4 중간의심 | 1) 암 의심 초음파 소견이 없는 저에코 고형결절 혹은 | 15–50 | 25 (15–34) | ≥1 cm |
| 2) 암 의심 초음파 소견이 있는 부분 낭성 혹은 등고에코 결절 | ||||
| 3 낮은의심 | 암 의심 초음파 소견이 없는 부분 낭성 혹은 등/고에코결절 | 3–15 | 8 (6–10) | ≥1.5 cm |
| 2 양성 | 1) 해면모양 | <3 | 0 | ≥2 cm |
| 2) comet tail artifact 보이는 부분 낭성 결절 혹은 순수 낭종 | <1 | 0 | ||
| 1 무결절 | – | – |
Table 3.
갑상선 세침흡인세포검사의 Bethesda system: 진단 범주와 악성도
Table 4.
Imaging-based risk stratification of the cervical lymph nodes for nodal metastasis48)
| Category | US | CT |
|---|---|---|
| Suspicious a | Cystic change | Cystic change |
| Calcification (micro/macro) | Calcification (micro/macro) | |
| Hyperechogenicity (focal or diffuse) | Heterogeneous enhancement | |
| Abnormal vascularity (peripheral or diffuse) | Strong enhancement (focal or diffuse) | |
| Indeterminate b | Loss of central hilar echo and absence of central hilar vascularity | Loss of central hilar fat and absence of central hilar vessel enhancement |
| Benign c | Central hilar echo | Central hilar fat |
| Central hilar vascularity | Central hilar vessel enhancement |
Table 5.
후두신경 기능 이상과 관련된 요소들
| 인자 | 증상과 징후 |
|---|---|
| 문진 | 음성 이상, 연하곤란, 기도 관련 증상, 객혈, 통증, 빠른 진행, 경부 또는 상흉부 수술 병력 |
| 이학적 검사 | 후두나 기관에 고정된 광범위하고 단단한 종물 |
| 영상학적 검사 | 후방 갑상선 외 침범 또는 기관식도 침범이 있는 경우, |
| 반회후두신경 또는 미주신경의 주행을 따라 광범위한 경부 림프절종이 있는 경우 |
Table 6.
갑상선암의 AJCC 지침(7판)에 따른 TNM 정의
Table 7.
갑상선유두암 또는 갑상선여포암의 병기
Table 8.
재발 위험도에 따른 환자의 분류
| 저위험군 | ▪유두암(아래 항목을 모두 충족하는 경우) |
| 1) 국소 및 원격전이가 없고 | |
| 2) 수술로 육안적 병소가 모두 제거되었으며 | |
| 3) 주위조직으로의 침윤이 없고 | |
| 4) 나쁜 예후를 갖는 조직형(키큰세포 변이종, 원주형세포 변이종, hobnail 변이종)이 아니며 | |
| 5) 방사성요오드 잔여갑상선제거술 이후에 시행한 첫 번째 치료 후 전신스캔에서 갑상선 부위(thyroid bed) 외에는 섭취가 없는 경우 | |
| 6) 혈관 침범이 없는 경우 | |
| 7) 림프절전이가 없거나 미세 림프절전이(<0.2 cm) 가 5개 이하인 경우∗ | |
| ▪갑상선 내에 국한된 피막에 둘러싸인 여포 변이종 유두암∗ | |
| ▪갑상선 내에 국한된 여포암의 경우 혈관 침범이 없거나 경미한(4부위 이하) 경우∗ | |
| ▪갑상선 내에 국한된 미세유두암(다발성이거나 BRAF V600E 돌연변이 양성 포함됨) ∗ | |
| 중간위험군 | 1) 수술 후 병리조직검사에서 갑상선 주위 연조직으로 현미경적 침윤 소견 |
| 2) 첫번째 방사성요오드 잔여갑상선제거술 후 전신스캔에서 갑상선 부위 이외의 경부 섭취가 있는 경우 | |
| 3) 원발 종양이 나쁜 예후를 갖는 조직형이거나, 혈관 침범 소견이 있는 경우 | |
| 4) 임상적으로 림프절전이가 있거나(clinical N1) 3 cm 미만 크기의 림프절전이(pathologic N1)가 5개를 초과하거나∗, | |
| 5) 갑상선 외 침범이 있고 BRAF V600E 돌연변이 양성인 다발성 미세유두암∗ | |
| 고위험군 | 1) 종양이 육안적으로 주위 조직을 침범하였거나, |
| 2) 종양을 완전히 제거하지 못하였거나, | |
| 3) 원격전이가 있거나 | |
| 4) 수술 후 혈중 갑상선글로불린 농도가 높아서 전이가 의심되거나 | |
| 5) 경부 림프절전이의 최대 직경이 3 cm 이상인 경우∗ | |
| 6) 광범위한 혈관 침범(4 부위 초과)이 있는 여포암∗ |
∗ 의 항목을 제외한 모든 항목들은 권고수준 1에 해당하며, ∗은 권고수준 3임
Table 9.
Clinical implications of response to therapy reclassification in patients with differentiated thyroid cancer treated with total thyroidectomy and radioiodine remnant ablation
Table 10.
키나아제 억제제 치료 연관 부작용에 대한 선별 권고
Table 11.
키나아제 억제제 치료를 시작하기 전에 확인할 사항




 PDF
PDF ePub
ePub Citation
Citation Print
Print


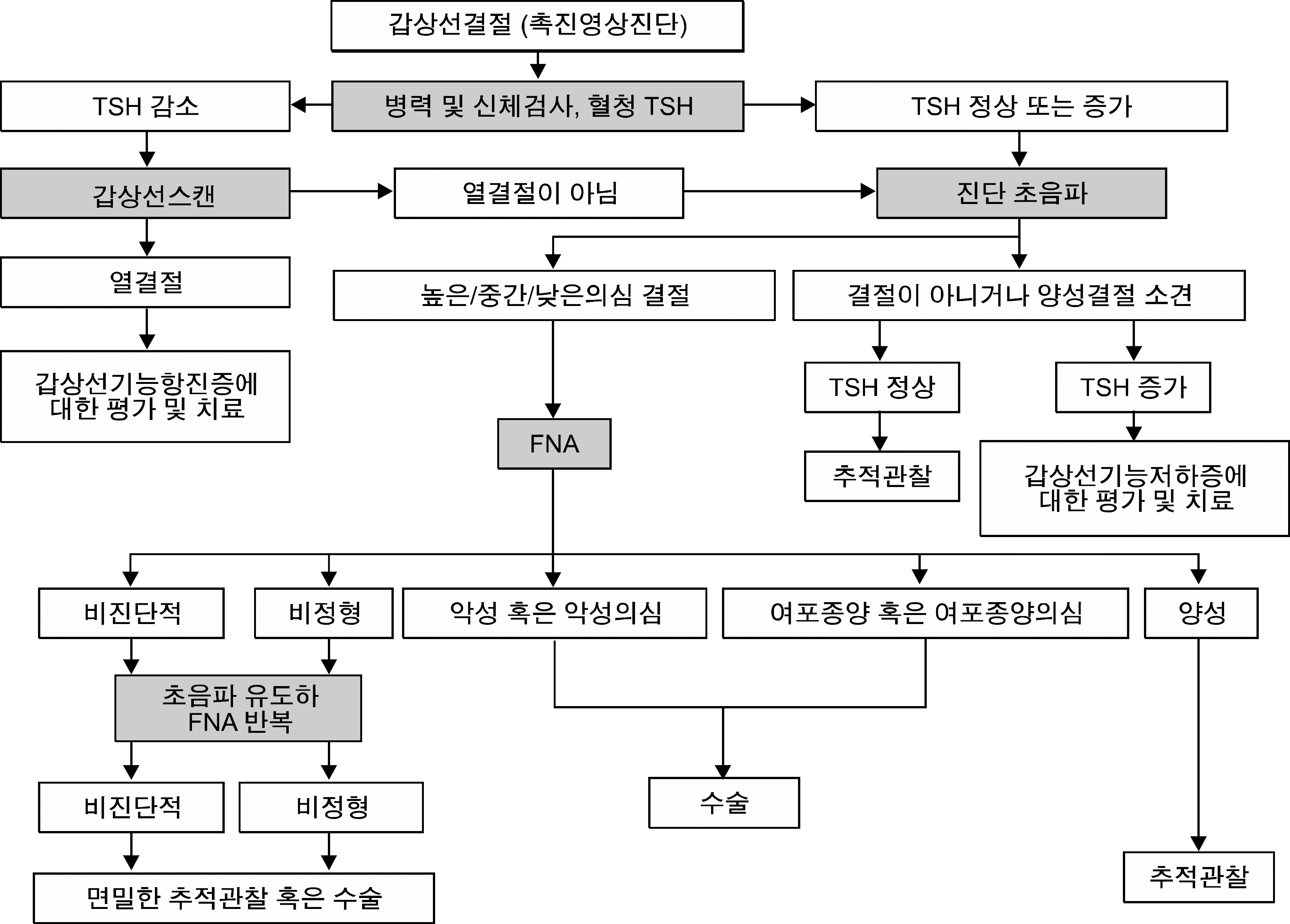
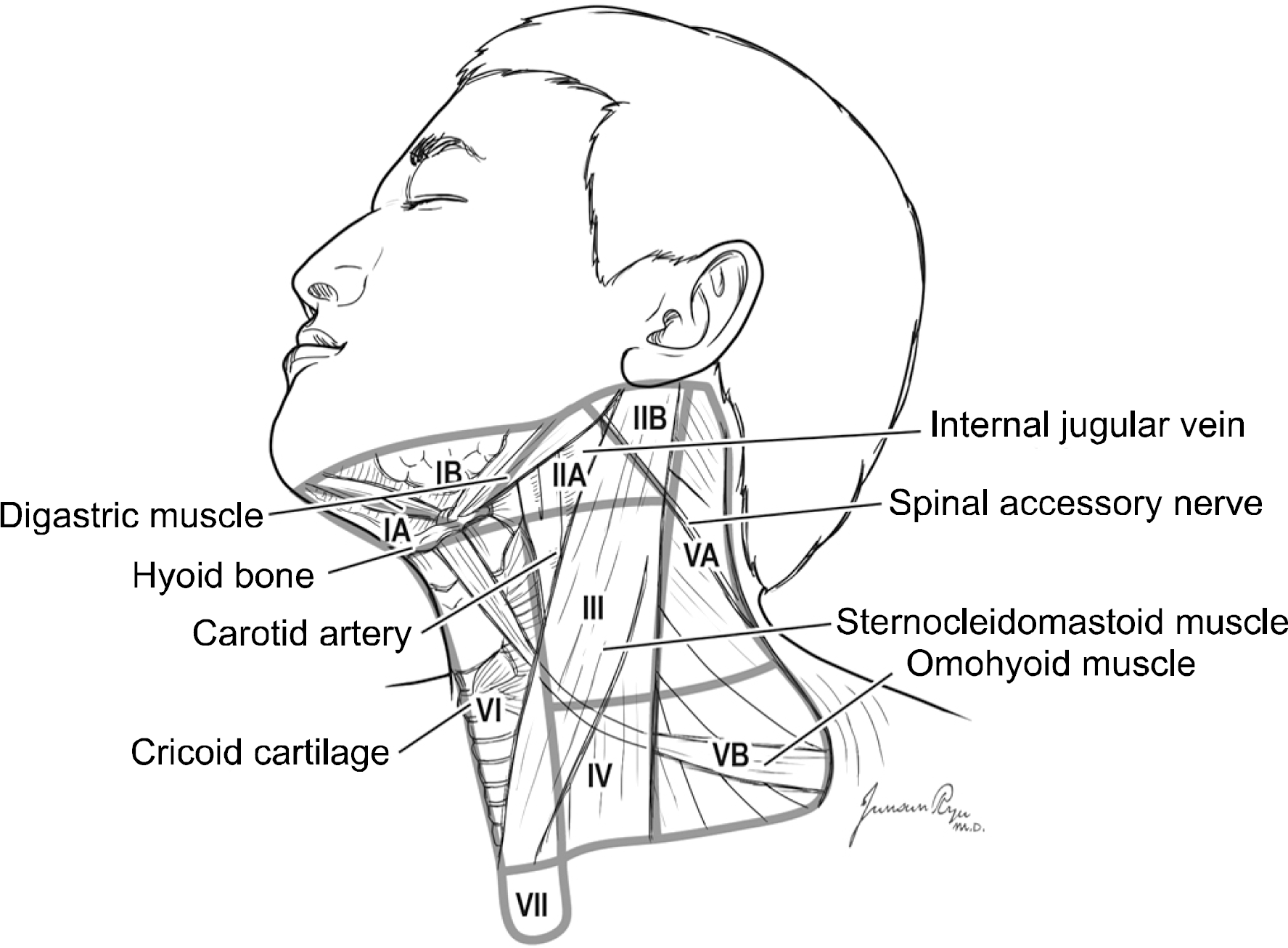
 XML Download
XML Download