Abstract
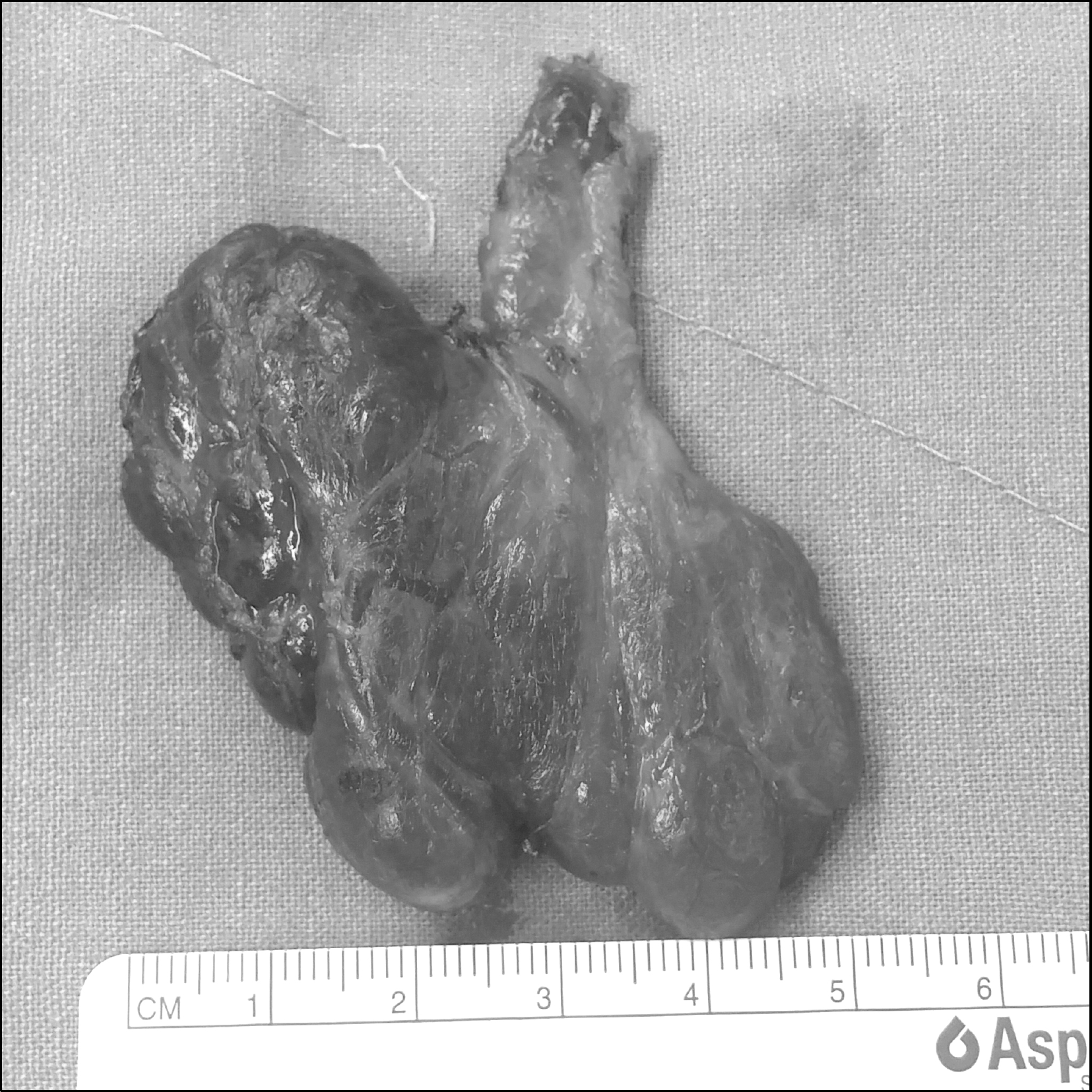
Thyroid hemiagenesis is a rare congenital anomaly that is caused by a developmental defect of a thyroid. Previous reports indicate that thyroid cancer associated with hemiagenesis is extremely rare. A 47-year-old woman presented with single nodule in the right thyroid gland that was incidentally detected during a routine medical checkup. Ultrasonography showed a 1.5×1.2 cm sized ill-defined irregular hypoehoic nodule in the right thyroid and the isthmus was present. However, the left thyroid was not seen and thyroid was disconnected at left paraisthmic area. Fine-needle aspiration cytology confirmed that the right thyroid nodule was papillary thyroid carcinoma. Total thyroidectomy with bilateral central compartment node dissection was performed. Permanent pathologic finding was 1.3×1 cm sized classical type papillary thyroid carcinoma with nodular hyperplasia. There was extensive lymphatic invasion and 3 metastatic lymph nodes out of 4 in central compartment. In conclusion, although thyroid hemiagenesis associated with thyroid carcinoma is extremely rare, treatment strategy is not different with patients with normal anatomy. And the possibility of developing a thyroid carcinoma should be considered in patients with hemiagenesis. Furthermore, it requires awareness of anatomical difference around the thyroid gland during operation.
References
1. Lipin R, Kandil E. Thyroid hemiagenesis. J La State Med Soc. 2012; 164(4):205–6.
3. Lee YS, Yun JS, Jeong JJ, Nam KH, Chung WY, Park CS. Thyroid hemiagenesis associated with thyroid adenomatous hyperplasia and papillary thyroid carcinoma. Thyroid. 2008; 18(3):381–2.

4. Pizzini AM, Papi G, Corrado S, Carani C, Roti E. Thyroid hemiagenesis and incidentally discovered papillary thyroid cancer: case report and review of the literature. J Endocrinol Invest. 2005; 28(1):66–71.

5. Wang J, Gao L, Song C. Thyroid hemiagenesis associated with medullary or papillary carcinoma: report of cases. Head Neck. 2014; 36(11):E106–11.

6. Maiorana R, Carta A, Floriddia G, Leonardi D, Buscema M, Sava L, et al. Thyroid hemiagenesis: prevalence in normal children and effect on thyroid function. J Clin Endocrinol Metab. 2003; 88(4):1534–6.

7. Shabana W, Delange F, Freson M, Osteaux M, De Schepper J. Prevalence of thyroid hemiagenesis: ultrasound screening in normal children. Eur J Pediatr. 2000; 159(6):456–8.

8. Huang SM, Chen HD, Wen TY, Kun MS. Right thyroid hemiagenesis associated with papillary thyroid cancer and an ectopic prelaryngeal thyroid: a case report. J Formos Med Assoc. 2002; 101(5):368–71.
9. Kim B, Kim IA. Means of monitoring the course of postoperative inflammatory processes in the abdominal cavity. Vestn Akad Med Nauk SSSR. 1981(6):46–8.
10. McHenry CR, Walfish PG, Rosen IB, Lawrence AM, Paloyan E. Congenital thyroid hemiagenesis. Am Surg. 1995; 61(7):634–8. ; discussion 8–9.
11. Khatri VP, Espinosa MH, Harada WA. Papillary adenocarcinoma in thyroid hemiagenesis. Head Neck. 1992; 14(4):312–5.

13. Park J-Y. Kim SJ, Cho YU. Thyroid hemiagenesis associated with micropapillary thyroid carcinoma. J Korean Surg Soc. 2010; 78(2):116–8.

14. Hamburger JI, Hamburger SW. Thyroidal hemiagenesis. Report of a case and comments on clinical ramifications. Arch Surg. 1970; 100(3):319–20.
16. Greening WP, Sarker SK, Osborne MP. Hemiagenesis of the thyroid gland. Br J Surg. 1980; 67(6):446–8.

17. Park SG, Ryu JW, Myung NH. Thyroid hemiagenesis and ectopic thymus at thyroid bed, and papillary cancer in opposite thyroid lobe with hyperthyroidism. J Korean Surg Soc. 2000; 58(3):433–7.
18. Nam YM, Park JS, Na KH, Ahn D. A case of thyroid hemiagenesis with concurrent papillary thyroid carcinoma. Korean J Otorhinolaryngol-Head Neck Surg. 2011; 54(8):557–9.

19. Karatag GY, Albayrak ZK, Onay HK, Karatag O, Peker O. Coexistence of thyroid hemiagenesis, nodular goitre and papillary carcinoma. Kulak Burun Bogaz Ihtis Derg. 2013; 23(2):115–8.

20. Campenni A, Giovinazzo S, Curto L, Giordano E, Trovato M, Ruggeri RM, et al. Thyroid hemiagenesis, Graves' disease and differentiated thyroid cancer: a very rare association: case report and review of literature. Hormones (Athens). 2015; 14(3):451–8.
Fig. 1.
Ultrasonography of the thyroid gland showing one hypoechoic nodule (arrow) in the right thyroid (A) and no thyroid tissue in the left thyroid (B).

Table 1.
Thyroid hemiagenesis associated with thyroid cancer




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download