Abstract
Background and Objectives
The relationship between Hashimoto thyroiditis (HT) and papillary thyroid cancer (PTC) is still controversial. Some studies suggested that molecular basis of the association between HT and PTC. BRAFV600E mutation is the most common genetic alteration founded in PTC. This study was to determine a role of BRAFV600E mutation in PTC with concurrent HT and their association with other clinicopathological factors.
Materials and Methods
We enrolled 452 patients who underwent thyroid surgery between 2009 and 2012 for classical PTC. The status of BRAFV600E mutation was evaluated by direct sequencing. HT was defined as presence of lymphocytic thyroiditis in pathology or positive serum anti-thyroid peroxidase antibody.
Results
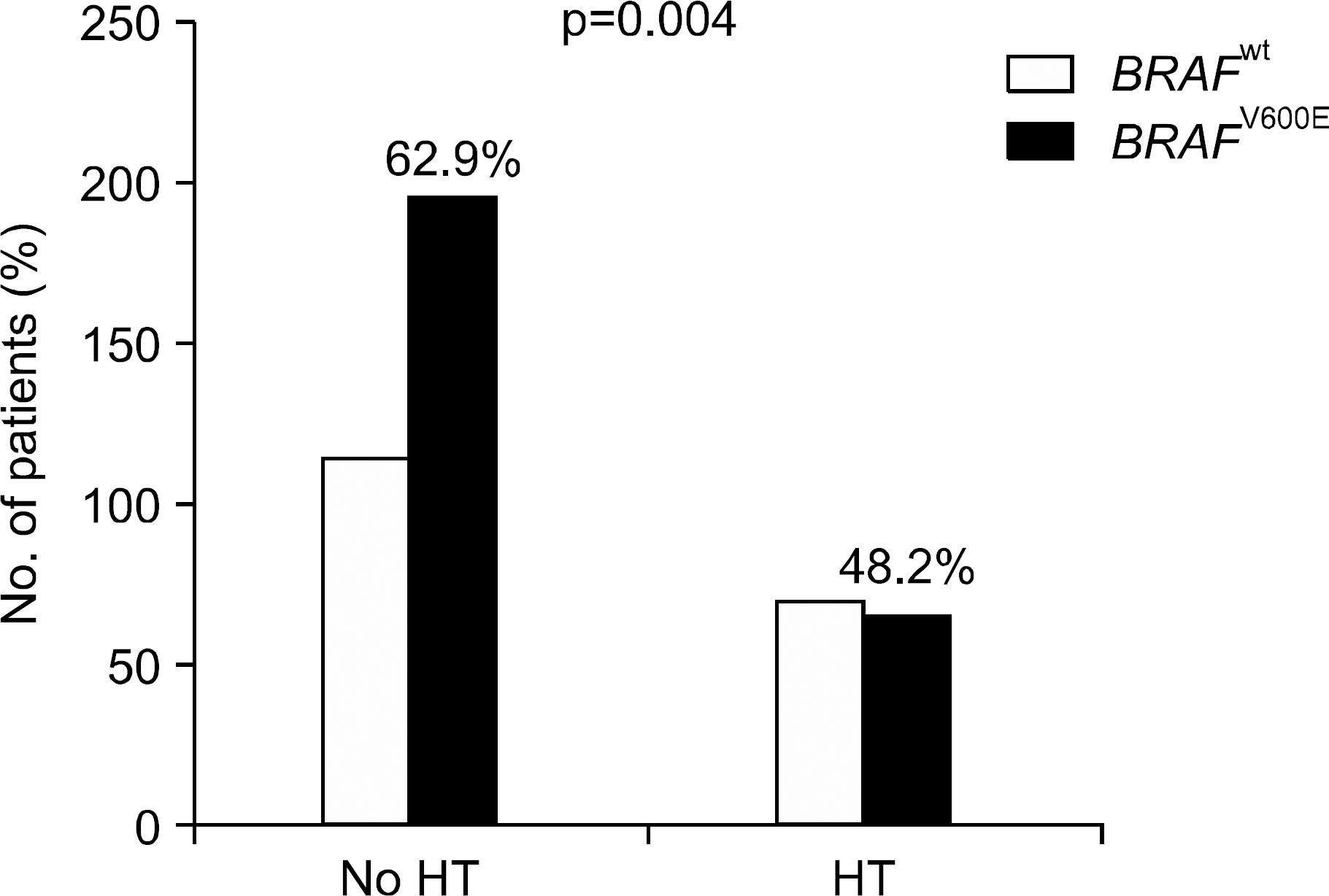
Total 139 patients (30%) with PTC had coexistence HT. HT was significantly associated female (p=0.006), and younger age (p=0.045). BRAFV600E mutation was confirmed in 264 patients (58%). The frequency of BRAFV600E mutation was significantly lower in PTC with coexistence HT (48.2%) compared by PTC without HT (62.9%, p=0.004). However, there was no significant difference in clinicopathological feature of PTC according to the presence of HT in patients with BRAFV600E mutated PTC. BRAFV600E mutation was less frequent in PTC with coexistence HT.
References
1. Kilfoy BA, Zheng T, Holford TR, Han X, Ward MH, Sjodin A, et al. International patterns and trends in thyroid cancer incidence, 1973–2002. Cancer Causes Control. 2009; 20(5):525–31.

2. Ahn HY, Park YJ. Incidence and clinical characteristics of thyroid cancer in Korea. Korean J Med. 2009; 77(5):537–42.
3. DeGroot LJ, Kaplan EL, McCormick M, Straus FH. Natural history, treatment, and course of papillary thyroid carcinoma. J Clin Endocrinol Metab. 1990; 71(2):414–24.

4. Staii A, Mirocha S, Todorova-Koteva K, Glinberg S, Jaume JC. Hashimoto thyroiditis is more frequent than expected when diagnosed by cytology which uncovers a pre-clinical state. Thyroid Res. 2010; 3(1):11.

5. Jankovic B, Le KT, Hershman JM. Clinical review: Hashimoto's thyroiditis and papillary thyroid carcinoma: is there a correlation? J Clin Endocrinol Metab. 2013; 98(2):474–82.
6. Lee JH, Kim Y, Choi JW, Kim YS. The association between papillary thyroid carcinoma and histologically proven Hashimoto's thyroiditis: a metaanalysis. Eur J Endocrinol. 2013; 168(3):): 343–9.

7. Prasad ML, Huang Y, Pellegata NS, de la Chapelle A, Kloos RT. Hashimoto's thyroiditis with papillary thyroid carcinoma (PTC)-like nuclear alterations express molecular markers of PTC. Histopathology. 2004; 45(1):39–46.

8. Di Pasquale M, Rothstein JL, Palazzo JP. Pathologic features of Hashimoto's-associated papillary thyroid carcinomas. Hum Pathol. 2001; 32(1):24–30.

9. Unger P, Ewart M, Wang BY, Gan L, Kohtz DS, Burstein DE. Expression of p63 in papillary thyroid carcinoma and in Hashimoto's thyroiditis: a pathobiologic link? Hum Pathol. 2003; 34(8):764–9.

10. Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002; 417(6892):949–54.

11. Xing M, Alzahrani AS, Carson KA, Shong YK, Kim TY, Viola D, et al. Association between BRAF V600E mutation and recurrence of papillary thyroid cancer. J Clin Oncol. 2015; 33(1):42–50.

12. Nucera C. Targeting thyroid cancer microenvironment: basic research and clinical applications. Front Endocrinol (Lausanne). 2013; 4:167.

13. Kim TY, Kim WB, Rhee YS, Song JY, Kim JM, Gong G, et al. The BRAF mutation is useful for prediction of clinical recurrence in low-risk patients with conventional papillary thyroid carcinoma. Clin Endocrinol (Oxf). 2006; 65(3):364–8.

14. Kim KH, Suh KS, Kang DW, Kang DY. Mutations of the BRAF gene in papillary thyroid carcinoma and in Hashimoto's thyroiditis. Pathol Int. 2005; 55(9):540–5.

15. Kim EY, Kim WG, Kim WB, Kim TY, Kim JM, Ryu JS, et al. Coexistence of chronic lymphocytic thyroiditis is associated with lower recurrence rates in patients with papillary thyroid carcinoma. Clin Endocrinol (Oxf). 2009; 71(4):581–6.

16. Choi YM, Kim WG, Kim TY, Bae SJ, Kim HK, Jang EK, et al. Low levels of serum vitamin D3 are associated with autoimmune thyroid disease in pre-menopausal women. Thyroid. 2014; 24(4):655–61.

17. Lee HJ, Choi J, Hwang TS, Shong YK, Hong SJ, Gong G. Detection of BRAF mutations in thyroid nodules by allele-specific PCR using a dual priming oligonucleotide system. Am J Clin Pathol. 2010; 133(5):802–8.
18. Ng WY, Lui KF, Thai AC, Cheah JS. Absence of ion channels CACN1AS and SCN4A mutations in thyrotoxic hypokalemic periodic paralysis. Thyroid. 2004; 14(3):187–90.

19. Singh B, Shaha AR, Trivedi H, Carew JF, Poluri A, Shah JP. Coexistent Hashimoto's thyroiditis with papillary thyroid carcinoma: impact on presentation, management, and outcome. Surgery. 1999; 126(6):1070–6. ; discussion 6–7.

20. Zhang L, Li H, Ji QH, Zhu YX, Wang ZY, Wang Y, et al. The clinical features of papillary thyroid cancer in Hashimoto's thyroiditis patients from an area with a high prevalence of Hashimoto's disease. BMC Cancer. 2012; 12:610.

22. McLeod DS, Watters KF, Carpenter AD, Ladenson PW, Cooper DS, Ding EL. Thyrotropin and thyroid cancer diagnosis: a systematic review and dose-response metaanalysis. J Clin Endocrinol Metab. 2012; 97(8):268292.

23. Powell DJ Jr, Russell J, Nibu K, Li G, Rhee E, Liao M, et al. The RET/PTC3 oncogene: metastatic solid-type papillary carcinomas in murine thyroids. Cancer Res. 1998; 58(23):5523–8.
24. Knauf JA, Sartor MA, Medvedovic M, Lundsmith E, Ryder M, Salzano M, et al. Progression of BRAF-induced thyroid cancer is associated with epithelial-mesenchymal transition requiring concomitant MAP kinase and TGFbeta signaling. Oncogene. 2011; 30(28):3153–62.
25. Knauf JA, Ma X, Smith EP, Zhang L, Mitsutake N, Liao XH, et al. Targeted expression of BRAFV600E in thyroid cells of transgenic mice results in papillary thyroid cancers that undergo dedifferentiation. Cancer Res. 2005; 65(10):423845.

26. Lee JH, Lee ES, Kim YS. Clinicopathologic significance of BRAF V600E mutation in papillary carcinomas of the thyroid: a metaanalysis. Cancer. 2007; 110(1):38–46.
27. Marotta V, Guerra A, Zatelli MC, Uberti ED, Di Stasi V, Faggiano A, et al. BRAF mutation positive papillary thyroid carcinoma is less advanced when Hashimoto's thyroiditis lymphocytic infiltration is present. Clin Endocrinol (Oxf). 2013; 79(5):733–8.
28. Kim SK, Song KH, Lim SD, Lim YC, Yoo YB, Kim JS, et al. Clinical and pathological features and the BRAF(V600E) mutation in patients with papillary thyroid carcinoma with and without concurrent Hashimoto thyroiditis. Thyroid. 2009; 19(2):137–41.

Table 1.
Clinical and pathological characteristics of patients with PTC according to the co-existence of HT
Table 2.
Univariate and multivariate analysis of the clinical and pathological factors associated with co-existence of HT in patients with classical PTC
Table 3.
Clinical and pathological characteristics of patients with BRAF V600E mutated PTC according to the co-existence of HT




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download