Abstract
Background and Objectives
The aim of this study was to determine whether pathologically proven central or lateral lymph node (LN) metastasis (pN1a or pN1b) could affect ablation success and recurrence after high-dose radioactive iodine (RAI) ablation. We also sought to identify the risk factors for long-term recurrence in patients with papillary thyroid carcinoma (PTC).
Materials and Methods
A total of 277 patients with pN1 disease who had undergone high-dose RAI ablation (5.55 GBq) between 2000 and 2006 were included in this retrospective study. We compared the ablation success rate and the recurrence rate between patients with pN1a and pN1b disease. Univariate and multivariate analyses were performed to identify the risk factors for recurrence.
Results
The median duration of follow-up was 10.2 years. The overall ablation success rate was 64%, and the ablation success rate in the pN1b group (49%) was lower than in the pN1a group (74%). The overall recurrence rate was 23%, and the recurrence rate in the pN1b group (30%) was higher than in pN1a group (18%). A higher ratio of metastatic LNs, a higher level of pre-ablation thyroglobulin, and ablation failure were significant risk factors for recurrence by multivariate analysis.
Conclusion
Patients with pN1b disease experienced a lower ablation success rate and a higher recurrence rate than patients with pN1a disease. However, a higher ratio of metastatic LNs, a higher level of pre-ablation thyroglobulin, and ablation failure were stronger risk factors than the pathological N stage for long term recurrence in patients with node-positive PTC.
References
1. Leboulleux S, Rubino C, Baudin E, Caillou B, Hartl DM, Bidart JM, et al. Prognostic factors for persistent or recurrent disease of papillary thyroid carcinoma with neck lymph node metastases and/or tumor extension beyond the thyroid capsule at initial diagnosis. J Clin Endocrinol Metab. 2005; 90(10):5723–9.

2. Smith VA, Sessions RB, Lentsch EJ. Cervical lymph node metastasis and papillary thyroid carcinoma: does the compartment involved affect survival? Experience from the SEER database. J Surg Oncol. 2012; 106(4):357–62.

3. The American Joint Committee on Cancer. Thyroid. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. editors.AJCC cancer staging manual. 7th ed.New York, NY: Springer;2010. p. 87–96.
4. American Thyroid Association Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009; 19(11):1167–214.

5. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016; 26(1):1–133.

6. Randolph GW, Duh QY, Heller KS, LiVolsi VA, Mandel SJ, Steward DL, et al. The prognostic significance of nodal metastases from papillary thyroid carcinoma can be stratified based on the size and number of metastatic lymph nodes, as well as the presence of extranodal extension. Thyroid. 2012; 22(11):1144–52.

7. Mazzaferri EL. Thyroid remnant 131I ablation for papillary and follicular thyroid carcinoma. Thyroid. 1997; 7(2):265–71.

8. Castagna MG, Cevenini G, Theodoropoulou A, Maino F, Memmo S, Claudia C, et al. Post-surgical thyroid ablation with low or high radioiodine activities results in similar outcomes in intermediate risk differentiated thyroid cancer patients. Eur J Endocrinol. 2013; 169(1):23–9.

9. de Meer SG, Dauwan M, de Keizer B, Valk GD, Borel Rinkes IH, Vriens MR. Not the number but the location of lymph nodes matters for recurrence rate and disease-free survival in patients with differentiated thyroid cancer. World J Surg. 2012; 36(6):1262–7.

10. Mallick U, Harmer C, Yap B, Wadsley J, Clarke S, Moss L, et al. Ablation with low-dose radioiodine and thyrotropin alfa in thyroid cancer. N Engl J Med. 2012; 366(18):1674–85.

11. Sabra MM, Grewal RK, Ghossein RA, Tuttle RM. Higher administered activities of radioactive iodine are associated with less structural persistent response in older, but not younger, papillary thyroid cancer patients with lateral neck lymph node metastases. Thyroid. 2014; 24(7):1088–95.

12. Pacini F, Ladenson PW, Schlumberger M, Driedger A, Luster M, Kloos RT, et al. Radioiodine ablation of thyroid remnants after preparation with recombinant human thyrotropin in differentiated thyroid carcinoma: results of an international, randomized, controlled study. J Clin Endocrinol Metab. 2006; 91(3):926–32.

13. Jeon MJ, Kim TY, Kim WG, Han JM, Jang EK, Choi YM, et al. Differentiating the location of cervical lymph node metastasis is very useful for estimating the risk of distant metastases in papillary thyroid carcinoma. Clin Endocrinol (Oxf). 2014; 81(4):593–9.

14. Nixon IJ, Wang LY, Palmer FL, Tuttle RM, Shaha AR, Shah JP, et al. The impact of nodal status on outcome in older patients with papillary thyroid cancer. Surgery. 2014; 156(1):137–46.

15. Beierwaltes WH, Rabbani R, Dmuchowski C, Lloyd RV, Eyre P, Mallette S. An analysis of "ablation of thyroid remnants" with I-131 in 511 patients from 1947–1984: experience at University of Michigan. J Nucl Med. 1984; 25(12):1287–93.
16. Schneider DF, Mazeh H, Chen H, Sippel RS. Lymph node ratio predicts recurrence in papillary thyroid cancer. Oncologist. 2013; 18(2):157–62.

17. Furtado Mde S, Rosario PW, Calsolari MR. Persistent and recurrent disease in patients with papillary thyroid carcinoma with clinically apparent (cN1), but not extensive, lymph node involvement and without other factors for poor prognosis. Arch Endocrinol Metab. 2015; 59(4):285–91.
18. Schlumberger M, Catargi B, Borget I, Deandreis D, Zerdoud S, Bridji B, et al. Strategies of radioiodine ablation in patients with low-risk thyroid cancer. N Engl J Med. 2012; 366(18):1663–73.

Fig. 1.
Comparison of ablation success rate between patients with papillary thyroid carcinoma who had pathological N1a and N1b disease.
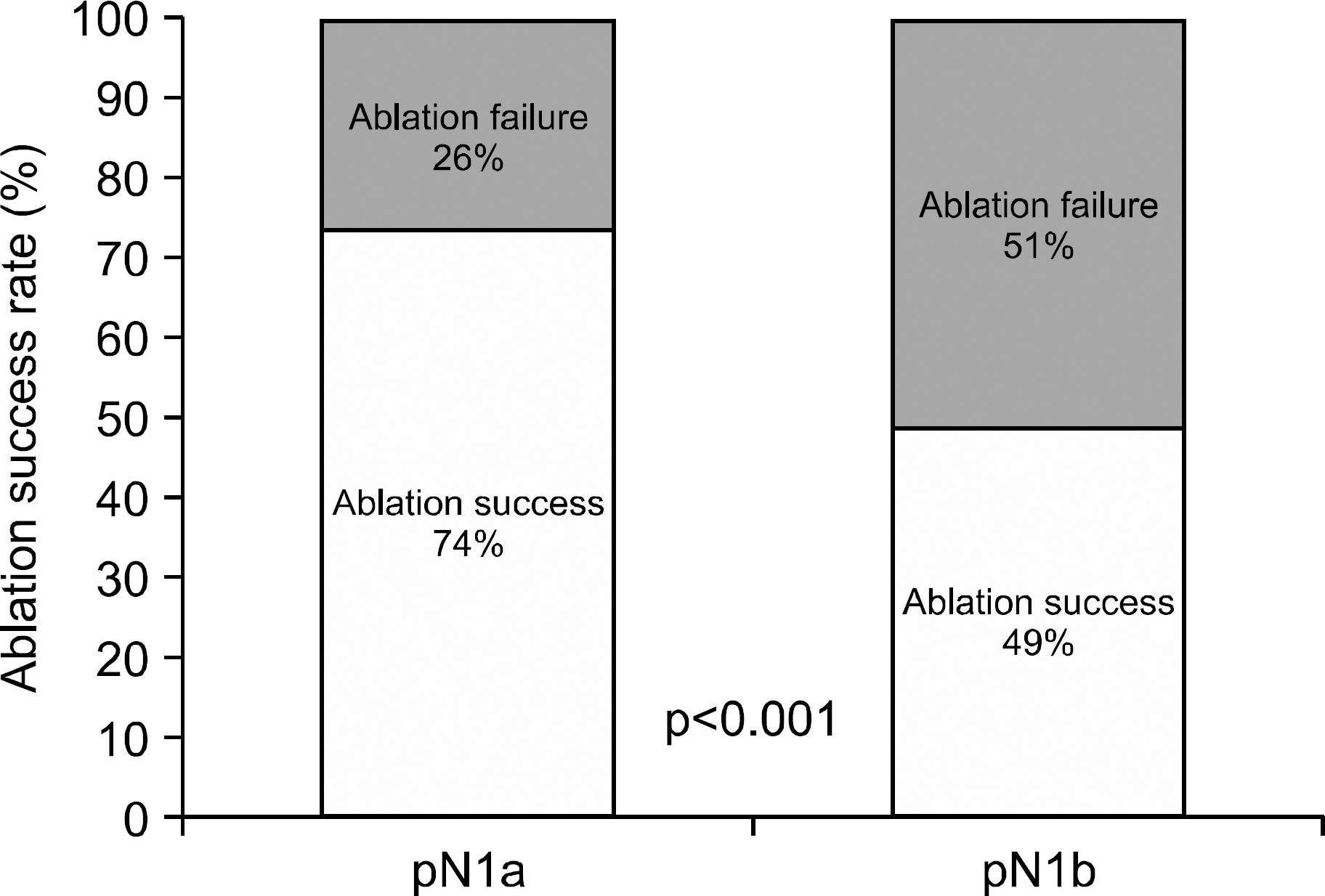
Fig. 2.
Comparison of recurrence rate between patients with papillary thyroid carcinoma who had pathological N1a and N1b disease.
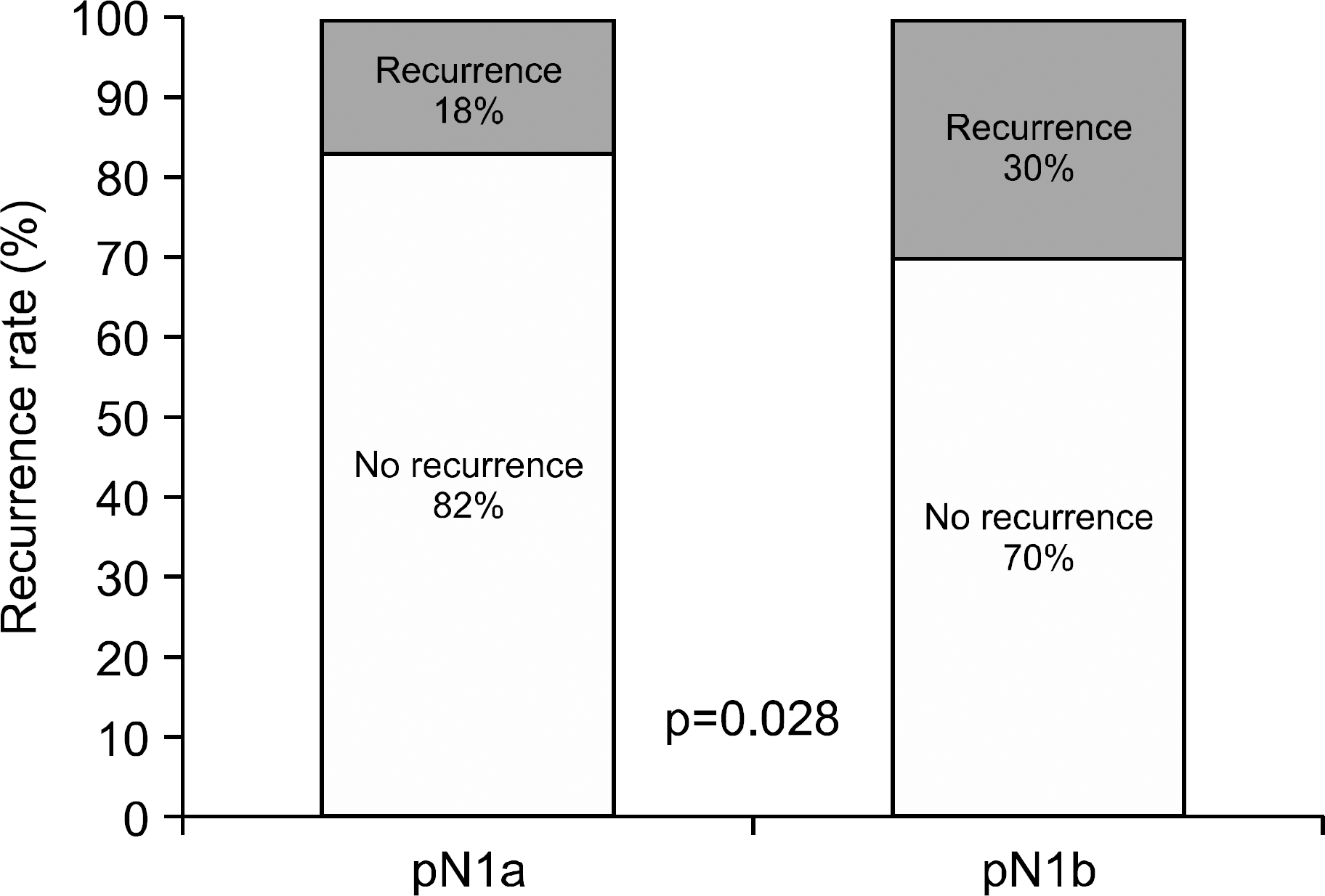
Fig. 3.
Kaplan-Meier survival analysis according to patholo-gical N1a and N1b disease in patients with papillary thyroid carcinoma.
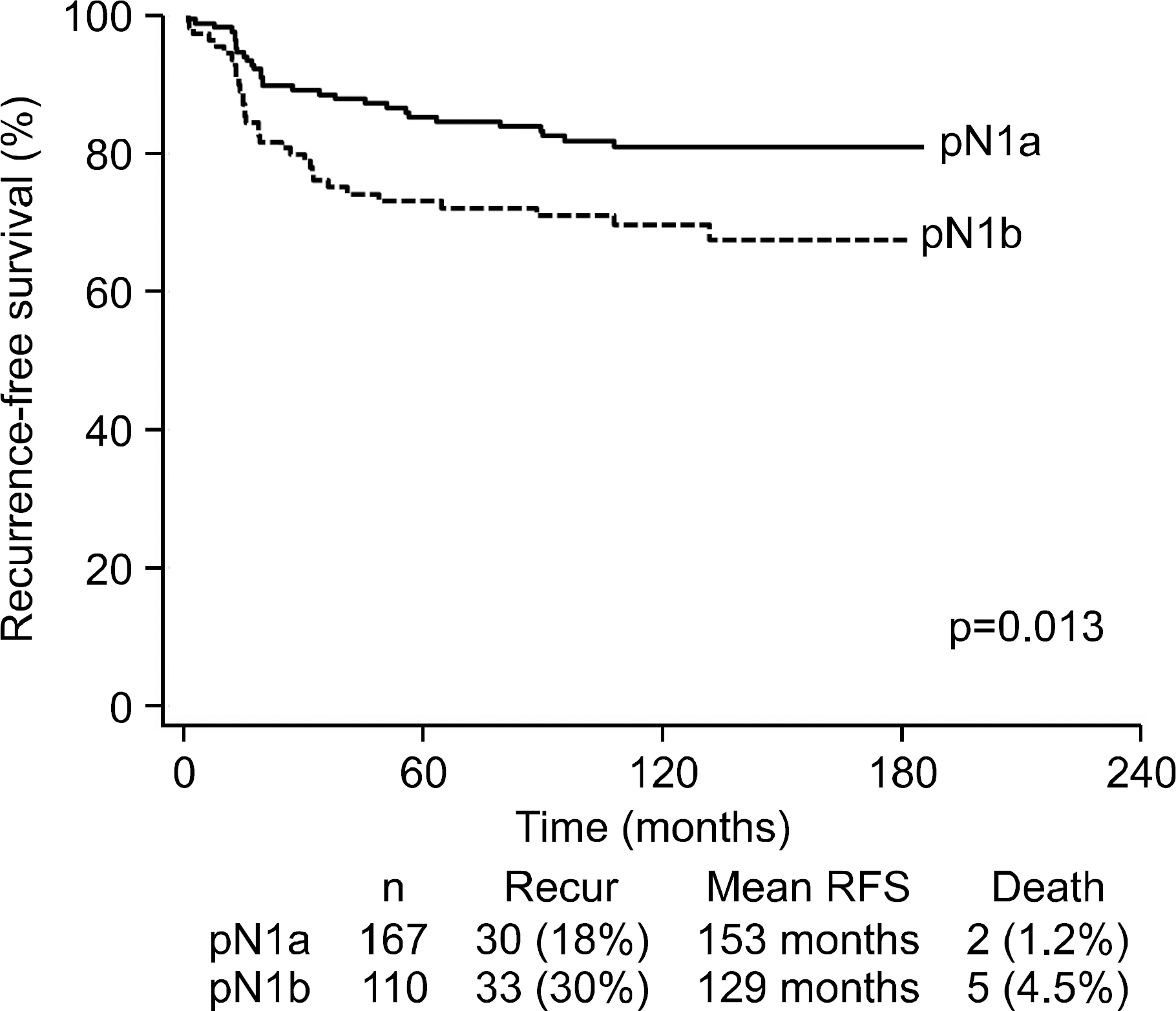
Table 1.
Patient characteristics
Table 2.
Univariate and multivariate analyses of clinicopathologic variables for recurrence-free survival in patients with node-positive papillary thyroid carcinoma




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download