Abstract
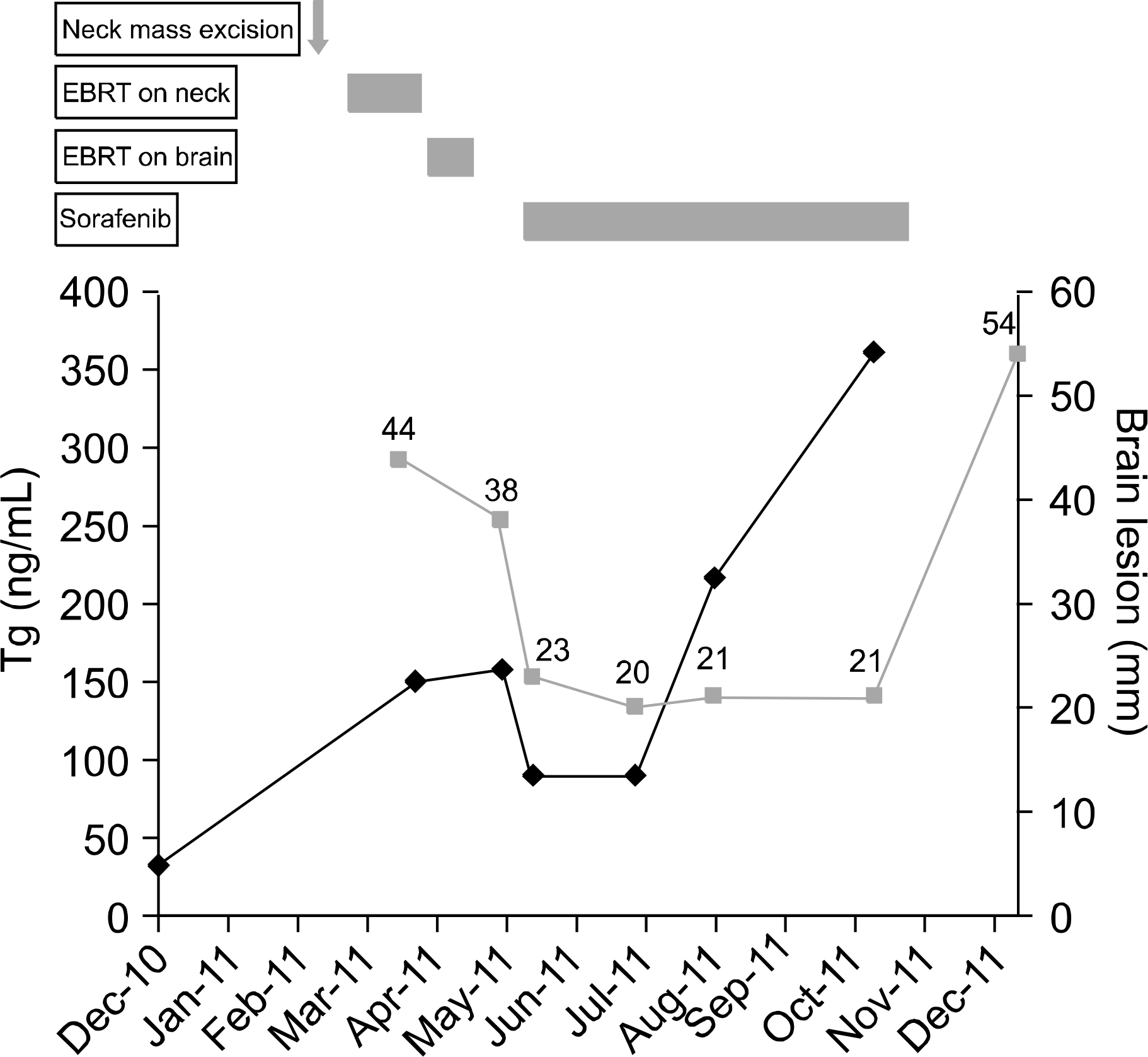
Sorafenib is an emerging therapeutic option for radioactive iodine (RAI)-refractory differentiated thyroid carcinoma. However, the effects of sorafenib as an adjuvant treatment following surgery or radiation on brain metastases from poorly differentiated thyroid carcinoma (PDTC) have never been reported. A 52-year-old patient underwent total thyroidectomy for follicular thyroid carcinoma. Despite high-dose RAI therapy, a neck mass and lung metastases were developed. PDTC was diagnosed by neck mass removal. During adjuvant external beam radiation therapy (EBRT) to the neck, brain metastases developed. After palliative EBRT for brain metastases, the brain tumor size decreased but lung metastases markedly progressed. Off-label sorafenib was used to treat progressive multiple metastatic lesions. Over five months of sorafenib treatment, the sum of the longest diameters for target lesions was decreased by 45% in brain and 13% in lung. Sorafenib can be considered a new adjuvant therapeutic option for metastatic brain lesions from PDTC after EBRT.
References
1. Ikekubo K, Hino M, Ito H, Hirao K, Ueshima M, Tanaka T, et al. Seven cases of brain metastasis from papillary thyroid carcinoma. Kaku Igaku. 2000; 37(4):349–57.
2. Henriques de Figueiredo B, Godbert Y, Soubeyran I, Carrat X, Lagarde P, Cazeau AL, et al. Brain metastases from thyroid carcinoma: a retrospective study of 21 patients. Thyroid. 2014; 24(2):270–6.

3. Sanders EM Jr, LiVolsi VA, Brierley J, Shin J, Randolph GW. An evidencebased review of poorly differentiated thyroid cancer. World J Surg. 2007; 31(5):934–45.

4. Hannallah J, Rose J, Guerrero MA. Comprehensive literature review: recent advances in diagnosing and managing patients with poorly differentiated thyroid carcinoma. Int J Endocrinol. 2013; 2013:317487.

5. Lyons JF, Wilhelm S, Hibner B, Bollag G. Discovery of a novel Raf kinase inhibitor. Endocr Relat Cancer. 2001; 8(3):219–25.

6. Xing M, Westra WH, Tufano RP, Cohen Y, Rosenbaum E, Rhoden KJ, et al. BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab. 2005; 90(12):6373–9.

7. Fenton C, Patel A, Dinauer C, Robie DK, Tuttle RM, Francis GL. The expression of vascular endothelial growth factor and the type 1 vascular endothelial growth factor receptor correlate with the size of papillary thyroid carcinoma in children and young adults. Thyroid. 2000; 10(4):349–57.

8. Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. 2014; 384(9940):319–28.

9. Kakudo K, Bai Y, Katayama S, Hirokawa M, Ito Y, Miyauchi A, et al. Classification of follicular cell tumors of the thyroid gland: analysis involving Japanese patients from one institute. Pathol Int. 2009; 59(6):359–67.

10. Patel KN, Shaha AR. Poorly differentiated thyroid cancer. Curr Opin Otolaryngol Head Neck Surg. 2014; 22(2):121–6.

11. McWilliams RR, Giannini C, Hay ID, Atkinson JL, Stafford SL, Buckner JC. Management of brain metastases from thyroid carcinoma: a study of 16 pathologically confirmed cases over 25 years. Cancer. 2003; 98(2):356–62.
12. Bernad DM, Sperduto PW, Souhami L, Jensen AW, Roberge D. Stereotactic radiosurgery in the management of brain metastases from primary thyroid cancers. J Neurooncol. 2010; 98(2):249–52.

13. Chen L, Shen Y, Luo Q, Yu Y, Lu H, Zhu R. Response to sorafenib at a low dose in patients with radioiodine-refractory pulmonary metastases from papillary thyroid carcinoma. Thyroid. 2011; 21(2):119–24.

14. Shen Y, Ruan M, Luo Q, Yu Y, Lu H, Zhu R, et al. Brain metastasis from follicular thyroid carcinoma: treatment with sorafenib. Thyroid. 2012; 22(8):856–60.

15. Krajewska J, Olczyk T, Roskosz J, Paliczk-Cieslik E, Smi-etana AK, Kaczmarek-Borowska B, et al. Treatment with sorafenib in advanced thyroid cancer – a case report. Endokrynol Pol. 2010; 61(5):492–6.
16. Lagas JS, van Waterschoot RA, Sparidans RW, Wagenaar E, Beijnen JH, Schinkel AH. Breast cancer resistance protein and P-glycoprotein limit sorafenib brain accumulation. Mol Cancer Ther. 2010; 9(2):319–26.

18. Girard N, Mornex F. Sorafenib and radiotherapy association for hepatocellular carcinoma. Cancer Radiother. 2011; 15(1):77–80.
Fig. 1.
Microscopic imaging of anterior neck mass. (A) The tumor shows a solid trabecular growth pattern and frequent necrosis (N) (H-E stain, ×40). (B) The tumor cells are monotonous and show small round nuclei with dark chromatin and frequent mitoses (arrows) (H-E stain, ×40).

Fig. 2.
Radiologic imaging of brain. (A) Brain metastases were diagnosed by MR ima-ging, showing a 3.8×4.4 cm mass with central necrosis in the right frontal lobe. (B) Shortly after EBRT for brain metastases. The dimensions of the mass had decreased to 3.8×2.7 cm. (C) Five mo-nths after sorafenib treat-ment. Brain CT showed de-crease in size of the mass (2.0×1.5 cm). (D) Two mon-ths after sorafenib disconti-nuation. Brain MRI showed marked progression of the mass (5.4×3.7 cm).
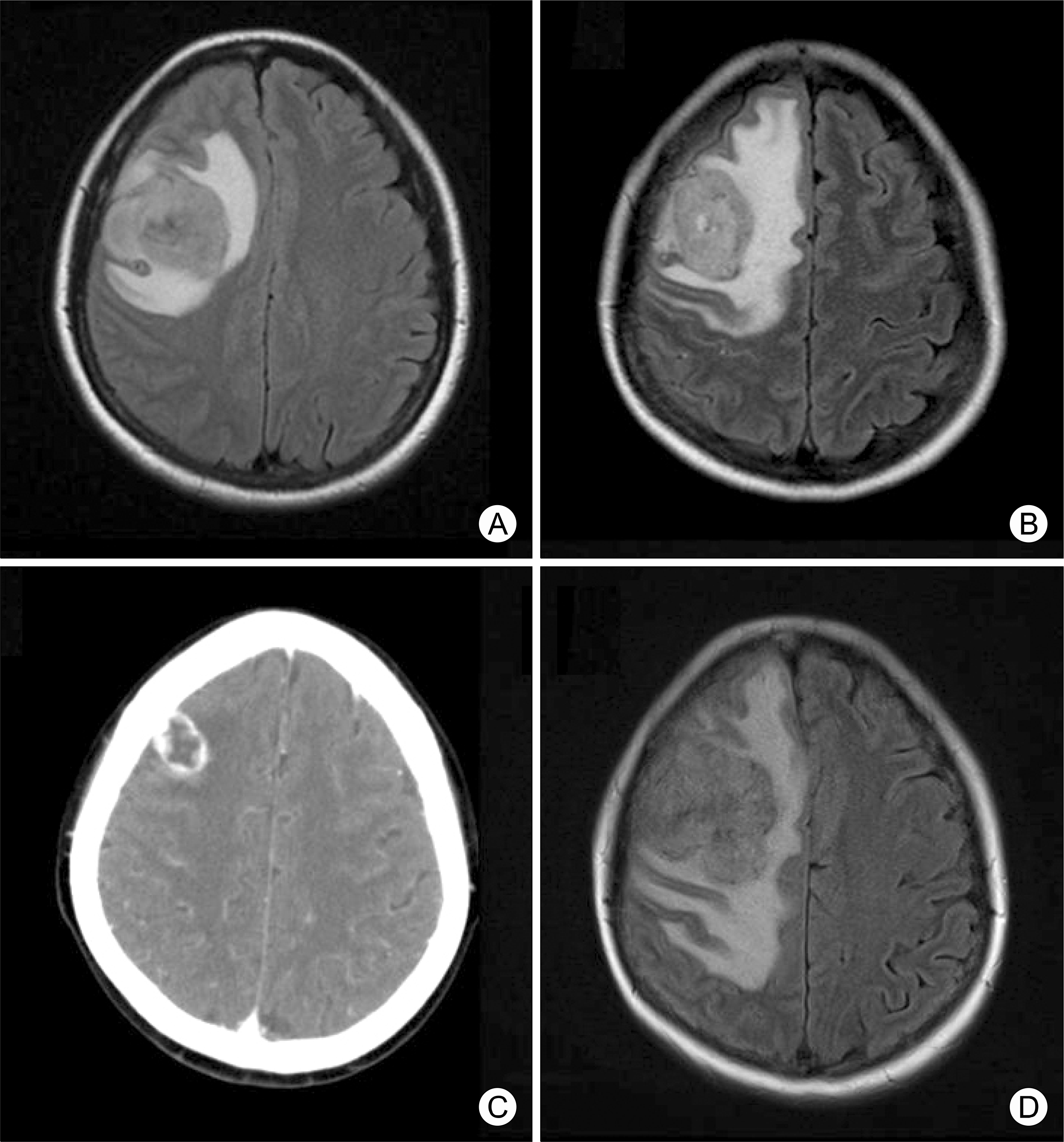
Fig. 3.
CT scan of the chest.(A) At the end of EBRT on thyroid bed. Chest CT ima-ges showed multiple meta-static nodules in both lungs. (B) Five months after sora-fenib initiation. Sum of the longest diameters of two no-dules decreased by 13%. (C) At the end of EBRT on thy-roid bed. Chest CT images showed no mediastinal lym-ph node enlargement. (D) Five months after sorafenib initiation. Mediastinal lymph nodes were extensively en-larged.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download