Abstract
Background and Objectives
The role of thyroid-stimulating hormone (TSH) signaling on osteoblastic differentiation is still undetermined. The aim of this study was to investigate the effects of 5-aza-2′-deoxycytidine (5-azacytidine) on TSH-mediated regulations of osteoblasts.
Materials and Methods
MG63, a human osteoblastic cell-line, was treated with 5-azacytidine before inducing osteogenic differentiation using osteogenic medium (OM) containing L-ascorbic acid and β-glyceophosphate. Bovine TSH or monoclonal TSH receptor stimulating antibody (TSAb) was treated. Quantitative real-time PCR analyses or measurement of alkaline phosphatase activities were performed for evaluating osteoblastic differentiation.
Results
Studies for osteogenic-related genes or alkaline phosphatase activity demonstrated that treatment of TSH or TSAb alone had no effects on osteoblastic differentiation in MG63 cells. However, treatment of 5-azacytidine, per se, significantly increased osteoblastic differentiation and combination treatment of 5-azacytidine and TSH or TSAb in the condition of OM showed further significant increase of osteoblastic differentiation.
Bone is one of the highly sensitive tissue to thyroid hormones, which have pivotal roles on fetal and childhood bone development, linear growth, and maintenance of adult bone homeostasis. Thyroid hormone deficiency in childhood results in growth retardation with bone maturation delays.1) In adults, thyrotoxicosis is one of the well-established cause of secondary osteoporosis, and recently an increased risk of hip fracture has been demonstrated in subclinical hyperthyroidism.2) Moreover, the fracture risk is also increased even thyroid hormonal status at the upper quadrant of the normal euthyroid reference range in postmenopausal women.3)
Apart the effects of the thyroid hormone, thyroid-stimulating hormone (TSH) signaling also has been suggested to have a role in bone metabolism. Intensive investigation of Tshr−/− mice and studies of intermittent TSH administration in mice and rat have demonstrated that TSH acts as a negative regulator of bone turnover.45) Still, the role of TSH in the skeletal homeostasis remains elusive. Its proposed actions are not entirely consistent with findings in human pathophysiologic status, animal models, and cellular experiments. One of the explanations is that it is hard to define the significance of TSH action independent from thyroid hormone in bone metabolism, since TSH and thyroid hormone move in reciprocal directions in vivo.
5-aza-2′-deoxycytidine (5-azacytidine) is a well-known demethylating agent. It has been reported that treatment of 5-azacytidine modulates thyroid-specific genes such as sodium and iodine symporter (NIS), thyroglobulin (Tg), thyroid peroxidase (TPO), and the thyroid-stimulating hormone receptor (TSHR). Especially, 5-azacytidine upregulated TSHR expressions in both normal thyroid epithelial cell and well-differentiated thyroid cancer cells.6) Interestingly, epigenetic regulations also modulate differentiation potentials in mesenchymal stem cells of osteoblasts. The aim of this study was to investigate the effects of 5-azacytidine on TSH-mediated regulations of osteoblasts.
Bovine TSH and 5-azacytidine were purchased from Sigma (St. Louis, MO, USA). M22 (a human monoclonal TSH receptor stimulating antibody, TSAb) was purchased from RSR Ltd (Cardiff, UK). Anti-b-catenin, anti-Smad antibodies were purchased from Cell signaling (Danvers, MA, USA).
A human osteoblast cell-line, MG63, was a kind gift from Professor Chan Soo Shin (Seoul National University College of Medicine, Seoul, Korea). Cells were culture in DMEM containing 10% FBS. For the osteogenic differentiation study, cells were plated in 12-well plates and mineralization was initiated at confluence with osteogenic medium (OM) including 50 µg/mL L-ascorbic acid and 10 mM β-glycerophosphate and the culture was performed for 7 days. Treatment of 5-azacytidine was treated every three other days, and TSH or TSAb was treated every 2 other days.
Total RNA was isolated from cells using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and subsequently reverse transcribed using the Reverse Transcription System kit (Promega, Madison, WI, USA). cDNA was amplified on an ABI Prism 7700 Sequence Detection System (Applied Biosystems, Foster City, CA, USA) with SYBR Green PCR technology. Rodent glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Applied Biosystems, Foster City, CA, USA) was used as an endogenous control. Relative quantification of the data was carried out using the standard curve method or the comparative cycle threshold (CT) method.7) Primers used are listed in Table 1. The relative gene expressions were measured to compare the ratios to the b-actin CT.
To assess the alkaline phosphatase (ALP) activity, cells were washed three times with ice-cold PBS (pH 7.4) and scraped immediately. Enzyme activity assays were performed in assay buffer (10 mM MgCl2 and 0.1 M alkaline buffer, pH 10.3) containing 10 mM p-nitrophenylphosphate as a substrate. The absorbance was read at optical density (OD) 405 nm. Relative ALP activity was defined as millimoles (mmol) of p-nitrophenol phosphate hydrolyzed per minute per milligram (mg) of total protein. ALP staining was carried out using an ALP kit according to the manufacturer's instructions (Promega, Southampton, UK).
For stimulating TSH signaling, bovine TSH or TSAb, M22 was treated to human osteoblastic cell-line (MG63) in osteogenic medium containing L-ascorbic acid and b-glycerophosphates. RT-PCR analyses demonstrated that there were no effects of treatment of TSH or M22 on mRNA expression of osteogenic marker gene, including ALP (Fig. 1A), Osteocalcin (Fig. 1B), and Collagen type I (Fig. 1C). Consistently, ALP activity was not changed with treatment of TSH or M22 (Fig. 1D). Furthermore, TSH or M22 was treated with major factors of osteogenic differentiation, Wnt-3a and bone morphogenetic protein (BMP)-2, and changes of their downstream signaling molecules, b-catenin and Smad proteins were analyzed by Western blot, respectively. Again, there were no effects of TSH or M22 treatment on it (Fig. 2A, B).
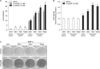
We then investigated the effects of TSH signaling on osteogenic differentiation in the presence of 5-azacytidine. Interestingly, treatment of 5-azacytidine, per se, increased ALP expression by 8.4-fold compared to control (5th vs. 1st column, p<0.01, Fig. 3A) and by 4.4-fold compared to OM group (5th vs. 2nd column, p<0.01, Fig. 3A). Treatment of 5-azacytidine in the presence of OM medium further increased ALP expression by 7.3-fold compared to control OM group (6th vs. 2nd column, p<0.01, Fig. 3A) and by 1.6-fold compared to 5-azacytidine with control medium group (6th vs. 5th column, p<0.01, Fig. 3A). Finally, treatment of TSH or TSAb further increased ALP gene expressions in condition of 5-azacytidine treatment, while it did not affect ALP gene expressions without 5-azacytidine. Moreover, the effects of TSH or TSAb were significantly greater in higher dose of 5-azacytidine (5 uM vs. 1 uM, Fig. 3A). In accordance with gene expression study, ALP activity assay and staining demonstrated that treatment of 5-azacytidine increased osteogenic differentiation (Fig. 3B, C). Treatment of TSH or TSAb increased osteogenic differentiation in the presence of 5-azacytidine, but not in absence of it (Fig. 3B, C).
Over recent years, evidences have been showing that TSH exerts direct effects on skeletal remodeling, independent of thyroid hormone, by interacting with specific receptors expressed on bone cells.8) In experimental animals, reduced expression of TSH receptor led to the osteoporosis development, inhibiting bone turnover.4) Moreover, administration of low doses of TSH in ovariectomized rats improved bone microstructure and prevented osteoporosis.5)
The anti-osteoclastogenic effects of TSH has been established. This suppressive action of TSH on osteoclast is mediated by reduced NF-κB and JNK signaling, and TNFα production.910) In Tshr−/− mice which showed low bone mass phenotype, TNFα production is upregulated in bone tissues.9) Since the genetic deletion of TNFα in these mice ameliorated low bone mass, as well as the bone formation and resorption defects, it is reasonable to deduce that the low bone mass phenotype of Tshr−/− mice is mediated by TNFα, at least in part.1011)
However, the role of TSH in osteoblast regulation is still undetermined. While it inhibits osteoblastic differentiations in bone marrow-derived mesenchymal stromal cell cultures, TSH stimulates differentiation and mineralization in murine cell cultures via a Wnt5a-dependent manner.12) Similarly, intermittently administered TSH showed anabolic effects on bone remodeling in both rats and mice.1314) Thus, the effect of TSH may be differentiation according to the differentiation stages. However, intermittent TSH injection inhibits ovariectomy-induced bone loss in rat model.14) Calcein-labeling demonstrates a direct anabolic action of intermittent TSH in this model.13) In humans, the titer of TSH receptor stimulating antibody demonstrated positive correlations with serum bone formation markers, bone specific ALP and osteocalcin, while TSH receptor blocking antibody showed negative correlations with osteocalcin in untreated Graves' patients, suggesting possible role of bone protective effects of TSH receptor blocking antibody.15)
Epigenetic controls such as DNA methylation or histone modification affect cellular differentiation in various progenitor cells and stem cells. Studies have been demonstrated that DNA methylation play roles in bone development16) and directly regulate osteogenic marker genes including, osteocalcin,17) Dlx and Osx.18) These finding led us to hypothesize that the effects of TSH signaling on osteoblastic differentiation, which is still in debates through several in vitro experimental studies, might be amplified and became conclusive in the condition of unmethylated status of osteoblast. Indeed, the present study clearly demonstrated that combination treatment of 5-azacytidine with TSH or TSAb significantly increased osteoblastic differentiation. The present study suggested that the independent effects of TSH signaling might be amplified in certain conditions which might enhance DNA unmethylation of osteoblasts. Further studies are needed to verify it.
In conclusion, stimulating TSH signaling has little effects on osteoblastic differentiation in vitro. However, in the condition of epigenetic modification using inhibitor of DNA methylation, TSH signaling positively affects osteoblastic differentiation in human osteoblasts. Epigenetic controls might direct the extrathyroidal effects of TSH signaling in various human pathophysiology.
Figures and Tables
Fig. 1
The effects of TSH signaling on osteogenic differentiation in MG63 cells. MG63 cells were treated with TSH (20 mIU/L) or monoclonal stimulatory TSHR antibody, M22 (TSAb, 10 ng/mL) with or without osteogenic medium containing L-ascorbic acid and b-glycerophosphate for 7 days. (A–C) mRNA expressions of (A) ALP, (B) OC, and (C) Col-I were detected using quantitative reverse transcription-polymerase chain reaction. (D) ALP activities were measured with each condition. ALP: alkaline phosphatase, Col-I: collagen type I, OC: osteocalcin. *p<0.05 vs. 1st column; #p<0.05 vs. 4th column.

Fig. 2
The effects of TSH signaling on Wnt/b-catenin and BMP-2/Smad signaling. (A) MG63 cells were treated with TSH (20 mIU/L) or monoclonal stimulatory TSHR antibody, M22 (TSAb, 10 ng/mL) with or without Wnt-3a for 3 days. Western blot analyses were performed using anti-b-catenin. (B) MG63 cells were treated with TSH (20 mIU/L) or monoclonal stimulatory TSHR antibody, M22 (TSAb, 10 ng/mL) with or without BMP-2 for 3 days. Western blot analyses were performed using anti-b-catenin.
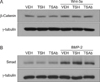
Fig. 3
The effects of combination treatment of 5-azacytidine and TSH on osteogenic differentiation. MG63 cells were treated with TSH (20 mIU/L) or monoclonal stimulatory TSHR antibody, M22 (TSAb, 10 ng/mL) with or without 5-azacytidine (1 or 5 µM) in osteogenic medium containing L-ascorbic acid and b-glycerophosphate for 4 days. (A) mRNA expressions of ALP were detected using quantitative reverse transcription-polymerase chain reaction. (B) ALP activities were quantified. (C) Staining of ALP activities was performed at 24-well plates. 5-azadC: 5-azacytidine, ALP: alkaline phosphatase, Col-I: collagen type I, OC: osteocalcin. *p<0.05 vs. 1st column; **p<0.01 vs. 1st column; #p<0.05 vs. 6th column.
Acknowledgments
This study was supported by a grant from the National R&D Program for Cancer Control, Ministry of Health and Welfare, Republic of Korea (No. 1720140).
References
1. Bassett JH, Williams GR. The molecular actions of thyroid hormone in bone. Trends Endocrinol Metab. 2003; 14(8):356–364.

2. Blum MR, Bauer DC, Collet TH, Fink HA, Cappola AR, da Costa BR, et al. Subclinical thyroid dysfunction and fracture risk: a meta-analysis. JAMA. 2015; 313(20):2055–2065.
3. Murphy E, Gluer CC, Reid DM, Felsenberg D, Roux C, Eastell R, et al. Thyroid function within the upper normal range is associated with reduced bone mineral density and an increased risk of nonvertebral fractures in healthy euthyroid postmenopausal women. J Clin Endocrinol Metab. 2010; 95(7):3173–3181.

4. Sun L, Davies TF, Blair HC, Abe E, Zaidi M. TSH and bone loss. Ann N Y Acad Sci. 2006; 1068:309–318.

5. Abe E, Marians RC, Yu W, Wu XB, Ando T, Li Y, et al. TSH is a negative regulator of skeletal remodeling. Cell. 2003; 115(2):151–162.

6. Tuncel M, Aydin D, Yaman E, Tazebay UH, Guc D, Dogan AL, et al. The comparative effects of gene modulators on thyroid-specific genes and radioiodine uptake. Cancer Biother Radiopharm. 2007; 22(3):443–449.

7. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008; 3(6):1101–1108.

9. Liu XS, Sajda P, Saha PK, Wehrli FW, Guo XE. Quantification of the roles of trabecular microarchitecture and trabecular type in determining the elastic modulus of human trabecular bone. J Bone Miner Res. 2006; 21(10):1608–1617.

10. Hase H, Ando T, Eldeiry L, Brebene A, Peng Y, Liu L, et al. TNFalpha mediates the skeletal effects of thyroid-stimulating hormone. Proc Natl Acad Sci U S A. 2006; 103(34):12849–12854.
11. Sun L, Zhu LL, Lu P, Yuen T, Li J, Ma R, et al. Genetic confirmation for a central role for TNFalpha in the direct action of thyroid stimulating hormone on the skeleton. Proc Natl Acad Sci U S A. 2013; 110(24):9891–9896.

12. Baliram R, Latif R, Berkowitz J, Frid S, Colaianni G, Sun L, et al. Thyroid-stimulating hormone induces a Wnt-dependent, feed-forward loop for osteoblastogenesis in embryonic stem cell cultures. Proc Natl Acad Sci U S A. 2011; 108(39):16277–16282.

13. Sampath TK, Simic P, Sendak R, Draca N, Bowe AE, O'Brien S, et al. Thyroid-stimulating hormone restores bone volume, microarchitecture, and strength in aged ovariectomized rats. J Bone Miner Res. 2007; 22(6):849–859.

14. Sun L, Vukicevic S, Baliram R, Yang G, Sendak R, McPherson J, et al. Intermittent recombinant TSH injections prevent ovariectomy-induced bone loss. Proc Natl Acad Sci U S A. 2008; 105(11):4289–4294.

15. Cho SW, Bae JH, Noh GW, Kim YA, Moon MK, Park KU, et al. The presence of thyroid-stimulation blocking antibody prevents high bone turnover in untreated premenopausal patients with Graves' disease. PLoS One. 2015; 10(12):e0144599.

16. de Andres MC, Kingham E, Imagawa K, Gonzalez A, Roach HI, Wilson DI, et al. Epigenetic regulation during fetal femur development: DNA methylation matters. PLoS One. 2013; 8(1):e54957.

17. Villagra A, Gutierrez J, Paredes R, Sierra J, Puchi M, Imschenetzky M, et al. Reduced CpG methylation is associated with transcriptional activation of the bone-specific rat osteocalcin gene in osteoblasts. J Cell Biochem. 2002; 85(1):112–122.

18. Lee JY, Lee YM, Kim MJ, Choi JY, Park EK, Kim SY, et al. Methylation of the mouse DIx5 and Osx gene promoters regulates cell type-specific gene expression. Mol Cells. 2006; 22(2):182–188.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download