Abstract
Background and Objectives
The level of thyroid-stimulating hormone (TSH)-stimulated thyroglobulin (Tg) after thyroid hormone withdrawal (THW) is the most sensitive marker for detecting recurrence of differentiated thyroid cancer (DTC). In DTC, Tg production is regulated by TSH; however, TSH values after THW are never identical, even in the same patient. The objective of this study was to evaluate the influence of TSH on Tg levels after THW.
Materials and Methods
TSH and Tg concentrations were measured twice at 2 and 3 weeks after THW in 309 patients with DTC. TSH and Tg levels at these time points were compared. The percent change in TSH (ΔTSH) and change in Tg level (%ΔTg) from 2 to 3 weeks after THW were calculated, and Pearson's correlation coefficients were calculated to determine whether ΔTSH could affect %ΔTg. Tg cutoff value for diagnostic imaging was 2 ng/mL.
Results
The TSH and Tg values at 3 weeks were significantly higher than those at 2 weeks after THW. Tg values increased significantly to >2 ng/mL after 1 week in 38.5% of the patients with Tg values of 0.2-2 ng/mL at 2 weeks after THW. In patients with Tg values ≥2 ng/mL at 2 weeks after THW, Tg values increased significantly after an additional week of THW. ΔTSH correlated significantly with %ΔTg.
thyroid carcinoma (DTC), the most common type of endocrine malignancy, has an excellent prognosis and 10-year survival rate higher than 90%.12 This outcome has been ensured by increased diagnostic scrutiny. However, the DTC recurrence rate is 10–30%, and the potential for reappearance after several years34) indicates the need for prolonged follow-up.
According to the American Thyroid Association guideline,5) a disease-free status comprises all of the following: no clinical evidence of tumor, no imaging evidence of tumor (i.e., no uptake outside the thyroid bed on the initial post-treatment whole-body scan or, if uptake outside the thyroid bed is present, no imaging evidence of tumor on a recent diagnostic scan and neck ultrasound), and undetectable serum thyroglobulin (Tg) values during thyroid-stimulating hormone (TSH) suppression and stimulation in the absence of interfering antibodies.
The TSH-stimulated Tg level after successful thyroid ablation is known as a sensitive and reliable marker for surveilling tumor persistence and recurrence,67) particularly in patients with low or undetectable Tg values in the absence of TSH stimulation. Frequently, stimulated Tg cutoff values of >1–2.5 ng/mL are used to detect or predict persistent disease.89101112) Although some recent studies have reported that Tg stimulation with recombinant human TSH may be more beneficial in patients who have undetectable (unstimulated) Tg values, thyroid hormone withdrawal (THW) is more frequently used to measure stimulated Tg.
The adequate TSH value needed to achieve sufficient Tg stimulation after THW has not been determined, and the commonly used TSH cutoff has been derived from the value considered necessary for radioactive iodine (RAI) imaging. Edmonds et al.13) reported a study of seven patients in whom a TSH value >30 µU/mL was necessary for adequate uptake on an RAI whole-body scan. Other groups have reported that a TSH value of >25–30 µU/mL at 2–3 weeks after THW is sufficient.1314151617) Valle et al.18) reported that TSH values of >80–100 µU/mL would serve as a more appropriate cutoff, compared to >30 µU/mL. Although many institutions apply a TSH cutoff of >30 µU/mL, it remains unclear when TSH or Tg values might plateau and whether Tg values would continue to rise once the TSH cutoff has been achieved. In addition, the same patient might exhibit different TSH values after each THW, even when an identical THW protocol has been applied consistently. We thought that if the TSH values differed between two serial follow-up tests, a comparison of Tg values without considering TSH might not be clinically relevant, given the possibility of a change in Tg levels due to different levels of TSH stimulation.
The aim of this study was to evaluate the influence of TSH levels on Tg and to determine whether we could predict the exact trend in Tg change between two serial follow-up tests in each patient.
Fig. 1 illustrates the THW protocol used in this study, including a change of levothyroxine to liothyronine for 3 weeks followed by a period of no thyroid hormone treatment for the next 2 weeks. From October 2008 through September 2013, we consecutively enrolled 4967 patients with DTC who underwent THW, and selected 652 (13.1%) with limited TSH elevation (<30 µU/mL) at 2 weeks after THW and repeated TSH measurement at 3 weeks after THW. Only patients with a TSH value >30 µU/mL at 3 weeks after THW were then selected to effectively demonstrate the influence of elevated TSH levels on Tg levels. Subjects with pituitary or hypothalamic disease were also excluded (Fig. 2). Finally, 309 patients were evaluated in this retrospective study, which was approved by the Institutional Review Board at our institution (Kyungpook National University Medical Center and School of Medicine, Daegu, Korea). The requirement for written informed consent by participants for the use of their clinical records in this study was waived because patient information was de-identified prior to analysis.
Radioimmunoassay commercial kits were used to measure serum Tg (Thyroglobulin IRMA; CIS Bio International, Gif sur Yvette, France). Seven calibrators, two controls, and patients' serum samples were mixed with buffer solution and incubated for 3 hours with agitation (400 rpm) at room temperature (18–25℃). The tubes were washed twice with washing solution, after which iodine-125–labeled anti-thyroglobulin was dispensed into each tube. The tubes were then incubated overnight (16–20 hours) at room temperature (18–25℃) without agitation. The tubes were again washed twice, and the remaining radioactivity bound to the tube was measured with a gamma scintillation counter for 1 minute. This Tg assay had a functional sensitivity of 0.7 ng/mL and analytical sensitivity of 0.2 ng/mL. The limit of detection for Tg in this study was ≥0.2 ng/mL.
Commercial immunoradiometric assay kits were used for TSH measurement (TSH IRMA, B·R·A·H·M·S GmbH, Hennigsdorf, Germany). Seven calibrators, two controls, and patients' serum samples were incubated with an excess of two anti-thyrotropin antibodies (mouse monoclonal) that recognized different binding sites on the antigen (TSH). One antibody was labeled with iodine-125, and the other was immobilized on the inner surface of the tube (coated tube system). The tubes were incubated in an orbital shaker (170–250 rpm) for 1 hour at room temperature (18–25℃), after which the remaining excess iodine-125–labeled anti-thyrotropin antibody was diluted and completely removed. The tubes were washed twice, and the radioactivity in each tube was measured for 1 minute in a gamma counter. The TSH assay had a functional sensitivity of 0.07 µU/mL.
For analysis, patients were divided into three groups according to their Tg values at 2 weeks after THW (Fig. 2): <0.2 ng/mL (108 patients, 35%), ≥0.2 to <2 ng/mL (148 patients, 48%), and ≥2 ng/mL (53 patients, 17%). The paired-samples t-test was used to compare differences in TSH and Tg levels from 2 to 3 weeks after THW in each patient. In patients with Tg values ≥2 ng/mL, the absolute change in TSH values (ΔTSH=TSH at 3 weeks after THW-TSH at 2 weeks after THW) and percent change in Tg values [%ΔTg=(Tg at 3 weeks THW−Tg at 2 weeks THW)/Tg at 2 weeks THW×100] from 2 to 3 weeks after THW were calculated, and Pearson's correlation coefficients were calculated to determine the relationship between ΔTSH and %ΔTg. A Tg cutoff value of 2 ng/mL was used to represent clinical significance for diagnostic imaging or further evaluations. MedCalc Statistical Software version 15.6.1 (MedCalc Software bvba, Ostend, Belgium) was used for statistical analysis. Continuous variables are presented as means±standard deviations (SDs). A p value <0.05 was considered to indicate a statistically significant difference.
The characteristics of all 309 patients are presented in Table 1. The study cohort included 41 men and 268 women with a mean age of 54.5±11.7 years. All patients underwent total or near-total thyroidectomy, which was completed by central neck dissection or modified radical neck dissection in 86% of cases, resulting in the apparent complete resection of the neoplastic tissue. In addition, 93% of patients underwent THW for ablation of the thyroid remnant or treatment of persistent thyroid cancer, and 7% underwent THW for thyroid cancer surveillance.
Fig. 3 and Supplementary Fig. 1 show the changes in serum Tg values in all 309 patients. In 227 patients (73.5%), the Tg values increased after an additional week of THW. The mean values of TSH and Tg at 2 weeks after THW were 16.9±7.7 µU/mL and 25.8±321.9 ng/mL, respectively, and the corresponding values at 3 weeks after THW were 56.6±14.3 µU/mL and 37.8±411.7 ng/mL, respectively. Tg values increased significantly after the additional week of THW (p=0.0233).
In a group of 108 patients with Tg values <0.2 ng/mL at 2 weeks after THW, the Tg values in approximately half (49.1%) of the patients remained below 0.2 ng/mL after the additional week of THW, whereas those in the other half (50.9%) increased but remained <2 ng/mL. Therefore, the Tg values at 3 weeks after THW had no clinical significance in patients with Tg values <0.2 ng/mL at 2 weeks after THW.
In a group of 148 patients with Tg values between 0.2 and 2 ng/mL at 2 weeks after THW, the Tg values in 121 (81.8%) patients increased after an additional week of THW, along with increases in TSH values (TSH: 19.1±8.0 to 55.1±13.8 µU/mL, p<0.0001; Tg: 0.5±0.3 to 2.9±3.2 ng/mL, p=0.0001). In these 121 patients, the Tg values of 57 (47.1%) patients increased to >2 ng/mL after an additional week of THW, accompanied by increases in TSH values (TSH: 15.0±7.6 to 56.8±14.5; Tg: 0.9±0.4 to 5.1±3.5, p<0.0001). We further dichotomized the 148 patients by their Tg values at 2 weeks after THW—122 patients with Tg values <1 ng/mL and 26 patients with Tg values ≥1 ng/mL—as a sub-analysis to determine the effect of Tg at 2 weeks after THW. Approximately 30% of 122 patients in the former group and approximately 77% of 26 patients in the latter eventually achieved a Tg value ≥2 ng/mL after the additional week. These results indicated that the patients with initially high Tg values achieved a relatively higher rate of increase in Tg after the additional week of THW (Table 2).
In a final group of 53 patients with Tg values ≥2 ng/mL, the Tg values of all but one patient remained in the same Tg category (≥2 ng/mL) after an additional week of THW (Fig. 4), and all but one of the 52 patients exhibited increased Tg values after the additional week (Table 3).
To evaluate the correlation between the changes in TSH and Tg values, we subjected 190 patients with detectable Tg values in both the first and second tests to a linear regression analysis and Pearson's correlation analysis. The %ΔTg was found to correlate positively with the ΔTSH (p=0.0001), with a correlation coefficient of 0.2881. We also performed the same analysis in the 53 patients with Tg values ≥2 ng/mL at 2 weeks after THW, a value that is suggestive of tum or recurrence or persistent tum or burden. The %ΔTg was also found to correlate positively with the ΔTSH (p=0.0001), with a correlation coefficient of 0.5037 (Fig. 5).
We performed a sub-analysis of 22 patients who underwent THW for thyroid cancer surveillance. The Tg values were undetectable in 12 (54.5%) of these 22 patients, and <2 ng/mL in the other 10 (45.5%) patients. Among the former 12 patients, three exhibited increased Tg values that remained <2 ng/mL after an additional week of THW. All but one of the latter 10 patients exhibited increased Tg values after the additional week of THW, and five (55.6%) of the nine patients' Tg values increased to >2 ng/mL after the additional week.
Generally, serum Tg values are used to predict successful thyroid remnant ablation,19) assess responses to RAI therapy,20) detect recurrent tumors,621) and predict disease recurrence2223) and prognosis.2425) A few studies have demonstrated that Tg values could increase in accordance with increases in TSH values.18)
The current large-scale study aimed to determine the importance of considering the serum TSH value with regard to Tg stimulation by THW. Our results demonstrated increased Tg values in a majority (73.5%) of patients after an additional week of THW. Furthermore, 38.5% of the patients with Tg values between 0.2 and 2 ng/mL at 2 weeks after THW exhibited increases to >2 ng/mL, suggesting a need for diagnostic imaging or other evaluations. These results are consistent with those of a previous report.18) The current study demonstrated that stimulated Tg levels in response to THW must be interpreted in the context of the corresponding TSH level to avoid drawing false conclusions about the disease status.
Almost all patients with Tg values >2 ng/mL at 2 weeks after THW demonstrated a significant increase in Tg along with an increase in TSH values after an additional week of THW. As Tg synthesis and secretion is an active process requiring stimulation by TSH, TSH values should be carefully considered by clinicians when determining whether Tg levels suggest the need for further diagnostic evaluations. Furthermore, our results also revealed that Tg values did not increase to >2 ng/mL at 3 weeks after THW in patients with undetectable Tg values at 2 weeks after THW. This result signifies that further clinical evaluation may not be needed when Tg values are undetectable at 2 weeks after THW without reference to TSH values.
A previous study18) tried to predict final Tg values using serial evaluations of each patient's Tg values at different time points. However, accurate prediction was not possible because the rate of increase was quite diverse for each patient. The residual tumor volumes always differed among patients; therefore, the rate of Tg increase in accordance with the increase of TSH value would always differ in each patient. We defined ΔTSH as the absolute change in TSH values and %ΔTg as the percent change in Tg values. Accordingly, using %ΔTg, we could overcome the difference of the rate of increase in each individual patient. Finally, %Δ Tg was found to correlated significantly with ΔTSH. This result indicates that Tg values should be interpreted in light of the TSH value in each individual patient at each individual testing time point.
Even among the 22 patients who underwent THW for thyroid cancer surveillance, nine of 10 patients with Tg values <2 ng/mL exhibited increased Tg values after the additional week of THW, and more than half of these nine patients' Tg values increased to >2 ng/mL. This result indicates that TSH levels should carefully be considered even among patients in the surveillance group, who were thought to be disease-free.
Meanwhile, the current study was limited by a THW duration of only 3 weeks. It remains unknown how long the TSH and Tg would continue to increase. Although they used a different THW protocol, Valle et al.18) reported that 4 weeks of THW was not sufficient to reach a final Tg value. The mean TSH values of the current study were lower than the range of 80–100 µU/mL suggested by their study. Nevertheless, in actual clinical situations, 3–4 weeks is a common interval for levothyroxine withdrawal. If levothyroxine is withdrawn for ≥4 weeks, liothyronine may be substituted; in such circumstances, 2–3 weeks is a common time period for liothyronine withdrawal. The current study did not assess the clinical significance of an increase in Tg values to >2 ng/mL at 3 weeks after THW. In future studies, we will investigate the clinical significance of a Tg increase after an additional week of THW.
In conclusion, the Tg value increases significantly in accordance with the increase in TSH values in patients with initially detectable Tg values. Careful consideration should be given to the TSH value when interpreting the meaning of changes in Tg levels in patients with DTC, as TSH values can always vary, even within the same patient and when the values exceed the cutoff (30 µU/mL), and can affect every measurement of stimulated Tg.
Figures and Tables
Fig. 2
Flowchart for patients with application of exclusion criteria. Tg: thyroglobulin, THW: thyroid hormone withdrawal, TSH: thyroid-stimulating hormone
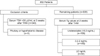
Fig. 3
Changes in serum thyroglobulin (Tg) values from 2 to 3 weeks after thyroid hormone withdrawal (THW) in all 309 patients.

Fig. 4
Pattern of changes in Tg values in accordance with the increase in the thyroid-stimulating hormone values in 53 patients with Tg values of >2 ng/mL at 2 weeks after thyroid hormone withdrawal (THW).

Fig. 5
Relationship between thyroglobulin (Tg) and thyroid-stimulating hormone (TSH) in 53 patients with a Tg value of >2 ng/mL at 2 weeks after thyroid hormone withdrawal.

Acknowledgments
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI15C0001).
References
1. Budak A, Gulhan I, Aldemir OS, Ileri A, Tekin E, Ozeren M. Lack of influence of pregnancy on the prognosis of survivors of thyroid cancer. Asian Pac J Cancer Prev. 2013; 14(11):6941–6943.

2. Lin Y, Li T, Liang J, Li X, Qiu L, Wang S, et al. Predictive value of preablation stimulated thyroglobulin and thyroglobulin/thyroid-stimulating hormone ratio in differentiated thyroid cancer. Clin Nucl Med. 2011; 36(12):1102–1105.

3. Mazzaferri EL. An overview of the management of papillary and follicular thyroid carcinoma. Thyroid. 1999; 9(5):421–427.

4. Mazzaferri EL, Kloos RT. Clinical review 128: Current approaches to primary therapy for papillary and follicular thyroid cancer. J Clin Endocrinol Metab. 2001; 86(4):1447–1463.

5. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009; 19(11):1167–1214.

6. Mazzaferri EL, Robbins RJ, Spencer CA, Braverman LE, Pacini F, Wartofsky L, et al. A consensus report of the role of serum thyroglobulin as a monitoring method for low-risk patients with papillary thyroid carcinoma. J Clin Endocrinol Metab. 2003; 88(4):1433–1441.

8. Brassard M, Borget I, Edet-Sanson A, Giraudet AL, Mundler O, Toubeau M, et al. Long-term follow-up of patients with papillary and follicular thyroid cancer: a prospective study on 715 patients. J Clin Endocrinol Metab. 2011; 96(5):1352–1359.

9. Haugen BR, Pacini F, Reiners C, Schlumberger M, Ladenson PW, Sherman SI, et al. A comparison of recombinant human thyrotropin and thyroid hormone withdrawal for the detection of thyroid remnant or cancer. J Clin Endocrinol Metab. 1999; 84(11):3877–3885.

10. Kloos RT. Thyroid cancer recurrence in patients clinically free of disease with undetectable or very low serum thyroglobulin values. J Clin Endocrinol Metab. 2010; 95(12):5241–5248.

11. Kloos RT, Mazzaferri EL. A single recombinant human thyrotropin-stimulated serum thyroglobulin measurement predicts differentiated thyroid carcinoma metastases three to five years later. J Clin Endocrinol Metab. 2005; 90(9):5047–5057.

12. Mazzaferri EL, Kloos RT. Is diagnostic iodine-131 scanning with recombinant human TSH useful in the follow-up of differentiated thyroid cancer after thyroid ablation? J Clin Endocrinol Metab. 2002; 87(4):1490–1498.

13. Edmonds CJ, Hayes S, Kermode JC, Thompson BD. Measurement of serum TSH and thyroid hormones in the management of treatment of thyroid carcinoma with radioiodine. Br J Radiol. 1977; 50(599):799–807.

14. Goldman JM, Line BR, Aamodt RL, Robbins J. Influence of triiodothyronine withdrawal time on 131I uptake postthyroidectomy for thyroid cancer. J Clin Endocrinol Metab. 1980; 50(4):734–739.

15. Hershman JM, Edwards CL. Serum thyrotropin (TSH) levels after thyroid ablation compared with TSH levels after exogenous bovine TSH: implications for 131-I treatment of thyroid carcinoma. J Clin Endocrinol Metab. 1972; 34(5):814–818.

16. Hilts SV, Hellman D, Anderson J, Woolfenden J, Van Antwerp J, Patton D. Serial TSH determination after T3 withdrawal or thyroidectomy in the therapy of thyroid carcinoma. J Nucl Med. 1979; 20(9):928–932.
17. Tamai H, Suemastu H, Kurokawa N, Esaki M, Ikemi T, Matsuzuka F, et al. Alterations in circulating thyroid hormones and thyrotropin after complete thyroidectomy. J Clin Endocrinol Metab. 1979; 48(1):54–58.

18. Valle LA, Gorodeski Baskin RL, Porter K, Sipos JA, Khawaja R, Ringel MD, et al. In thyroidectomized patients with thyroid cancer, a serum thyrotropin of 30 muU/mL after thyroxine withdrawal is not always adequate for detecting an elevated stimulated serum thyroglobulin. Thyroid. 2013; 23(2):185–193.

19. Kendler DB, Vaisman F, Corbo R, Martins R, Vaisman M. Preablation stimulated thyroglobulin is a good predictor of successful ablation in patients with differentiated thyroid cancer. Clin Nucl Med. 2012; 37(6):545–549.

20. Tamilia M, Al-Kahtani N, Rochon L, Hier MP, Payne RJ, Holcroft CA, et al. Serum thyroglobulin predicts thyroid remnant ablation failure with 30 mCi iodine-131 treatment in patients with papillary thyroid carcinoma. Nucl Med Commun. 2011; 32(3):212–220.

21. Eustatia-Rutten CF, Smit JW, Romijn JA, van der Kleij-Corssmit EP, Pereira AM, Stokkel MP, et al. Diagnostic value of serum thyroglobulin measurements in the follow-up of differentiated thyroid carcinoma, a structured meta-analysis. Clin Endocrinol (Oxf). 2004; 61(1):61–74.

22. Pelttari H, Valimaki MJ, Loyttyniemi E, Schalin-Jantti C. Post-ablative serum thyroglobulin is an independent predictor of recurrence in low-risk differentiated thyroid carcinoma: a 16-year follow-up study. Eur J Endocrinol. 2010; 163(5):757–763.

23. Toubeau M, Touzery C, Arveux P, Chaplain G, Vaillant G, Berriolo A, et al. Predictive value for disease progression of serum thyroglobulin levels measured in the postoperative period and after (131)I ablation therapy in patients with differentiated thyroid cancer. J Nucl Med. 2004; 45(6):988–994.
24. Lin JD, Huang MJ, Hsu BR, Chao TC, Hsueh C, Liu FH, et al. Significance of postoperative serum thyroglobulin levels in patients with papillary and follicular thyroid carcinomas. J Surg Oncol. 2002; 80(1):45–51.

25. Webb RC, Howard RS, Stojadinovic A, Gaitonde DY, Wallace MK, Ahmed J, et al. The utility of serum thyroglobulin measurement at the time of remnant ablation for predicting disease-free status in patients with differentiated thyroid cancer: a meta-analysis involving 3947 patients. J Clin Endocrinol Metab. 2012; 97(8):2754–2763.





 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download