초록
Background and Objectives
Thyroid dysfunction during pregnancy can result in many complications for both mother and infant. Due to the physiologic changes in thyroid stimulating hormone (TSH) level during early pregnancy, it is recommend to use trimester-specific reference ranges for every population. We obtained the reference range of TSH during the first trimester in Korean women according to gestational week.
Materials and Methods
The study population consisted of pregnant women who had undergone a TSH screening during the first trimester of pregnancy (n=8365) and nonpregnant women (n=1835).
Results
Median concentration of serum TSH decreased significantly from the 5th to 8th week of gestation (median TSH concentration: 2.00 mIU/L for 5 weeks; 1.70 mIU/L for 6 weeks; 1.40 mIU/L for 7 weeks; 1.05 mIU/L for 8 weeks). However, there was no significant difference in median concentration of serum TSH from the 8th to 12th weeks of gestation. Using the fixed cutoff value of TSH >3.66 mIU/L, the diagnosis rate of subclinical hypothyroidism was 15.0% for 5 weeks, 10.0% for 6 weeks, 5.9% for 7 weeks, and 3.6% for 8-12 weeks.
REFERENCES
1). Stagnaro-Green A. Overt hyperthyroidism and hypothyroidism during pregnancy. Clin Obstet Gynecol. 2011; 54(3):478–87.

3). Vaidya B, Anthony S, Bilous M, Shields B, Drury J, Hutchison S, et al. Detection of thyroid dysfunction in early pregnancy: Universal screening or targeted high-risk case finding? J Clin Endocrinol Metab. 2007; 92(1):203–7.

4). Horacek J, Spitalnikova S, Dlabalova B, Malirova E, Vizda J, Svilias I, et al. Universal screening detects two-times more thyroid disorders in early pregnancy than targeted high-risk case finding. Eur J Endocrinol. 2010; 163(4):645–50.

5). Glinoer D, Spencer CA. Serum TSH determinations in pregnancy: how, when and why? Nat Rev Endocrinol. 2010; 6(9):526–9.

6). Stagnaro-Green A, Abalovich M, Alexander E, Azizi F, Mestman J, Negro R, et al. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2011; 21(10):1081–125.

7). De Groot L, Abalovich M, Alexander EK, Amino N, Barbour L, Cobin RH, et al. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012; 97(8):2543–65.

8). Lazarus J, Brown RS, Daumerie C, Hubalewska-Dydejczyk A, Negro R, Vaidya B. 2014 European Thyroid Association guidelines for the management of subclinical hypothyroidism in pregnancy and in children. Eur Thyroid J. 2014; 3(2):76–94.

9). Li C, Shan Z, Mao J, Wang W, Xie X, Zhou W, et al. Assessment of thyroid function during first-trimester pregnancy: what is the rational upper limit of serum TSH during the first trimester in Chinese pregnant women? J Clin Endocrinol Metab. 2014; 99(1):73–9.

10). Blatt AJ, Nakamoto JM, Kaufman HW. National status of testing for hypothyroidism during pregnancy and postpartum. J Clin Endocrinol Metab. 2012; 97(3):777–84.

11). Stagnaro-Green A. Postpartum management of women begun on levothyroxine during pregnancy. Front Endocrinol (Lausanne). 2015; 6:183.

12). Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. 2017; 27(3):315–89.

13). Klein RZ, Haddow JE, Faix JD, Brown RS, Hermos RJ, Pulkkinen A, et al. Prevalence of thyroid deficiency in pregnant women. Clin Endocrinol (Oxf). 1991; 35(1):41–6.

14). Casey BM, Dashe JS, Wells CE, McIntire DD, Byrd W, Leveno KJ, et al. Subclinical hypothyroidism and pregnancy outcomes. Obstet Gynecol. 2005; 105(2):239–45.

15). Glinoer D. The regulation of thyroid function in pregnancy: pathways of endocrine adaptation from physiology to pathology. Endocr Rev. 1997; 18(3):404–33.

16). Maraka S, Ospina NM, O'Keeffe DT, Espinosa De Ycaza AE, Gionfriddo MR, Erwin PJ, et al. Subclinical hypothyroidism in pregnancy: a systematic review and metaanalysis. Thyroid. 2016; 26(4):580–90.

17). Glinoer D. Thyroid hyperfunction during pregnancy. Thyroid 1998;8(9). 859–64.
18). Ballabio M, Poshychinda M, Ekins RP. Pregnancy-induced changes in thyroid function: role of human chorionic gonadotro-pin as putative regulator of maternal thyroid. J Clin Endocrinol Metab. 1991; 73(4):824–31.

19). Medici M, Korevaar TI, Visser WE, Visser TJ, Peeters RP. Thyroid function in pregnancy: what is normal? Clin Chem. 2015; 61(5):704–13.

20). McNeil AR, Stanford PE. Reporting thyroid function tests in pregnancy. Clin Biochem Rev. 2015; 36(4):109–26.
21). Negro R, Schwartz A, Gismondi R, Tinelli A, Mangieri T, Stagnaro-Green A. Universal screening versus case finding for detection and treatment of thyroid hormonal dysfunction during pregnancy. J Clin Endocrinol Metab. 2010; 95(4):1699–707.

22). Li Y, Shan Z, Teng W, Yu X, Li Y, Fan C, et al. Abnormalities of maternal thyroid function during pregnancy affect neuropsychological development of their children at 25-30 months. Clin Endocrinol (Oxf). 2010; 72(6):825–9.
23). Su PY, Huang K, Hao JH, Xu YQ, Yan SQ, Li T, et al. Maternal thyroid function in the first twenty weeks of pregnancy and subsequent fetal and infant development: a prospective population-based cohort study in China. J Clin Endocrinol Metab. 2011; 96(10):3234–41.

24). Bryant SN, Nelson DB, McIntire DD, Casey BM, Cunningham FG. An analysis of population-based prenatal screening for overt hypothyroidism. Am J Obstet Gynecol. 2015; 213(4):565 e1-6.

25). Laurberg P, Andersen SL, Hindersson P, Nohr EA, Olsen J. Dynamics and predictors of serum TSH and fT4 reference limits in early pregnancy: a study within the Danish National Birth Cohort. J Clin Endocrinol Metab. 2016; 101(6):2484–92.

26). Kim HS, Kim BJ, Oh S, Lee da Y, Hwang KR, Jeon HW, et al. Gestational age-specific cutoff values are needed for diagnosis of subclinical hypothyroidism in early pregnancy. J Korean Med Sci. 2015; 30(9):1308–12.

Fig. 1.
Serum thyroid stimulating hormone variation according to gestational week in 8365 Korean pregnant women.
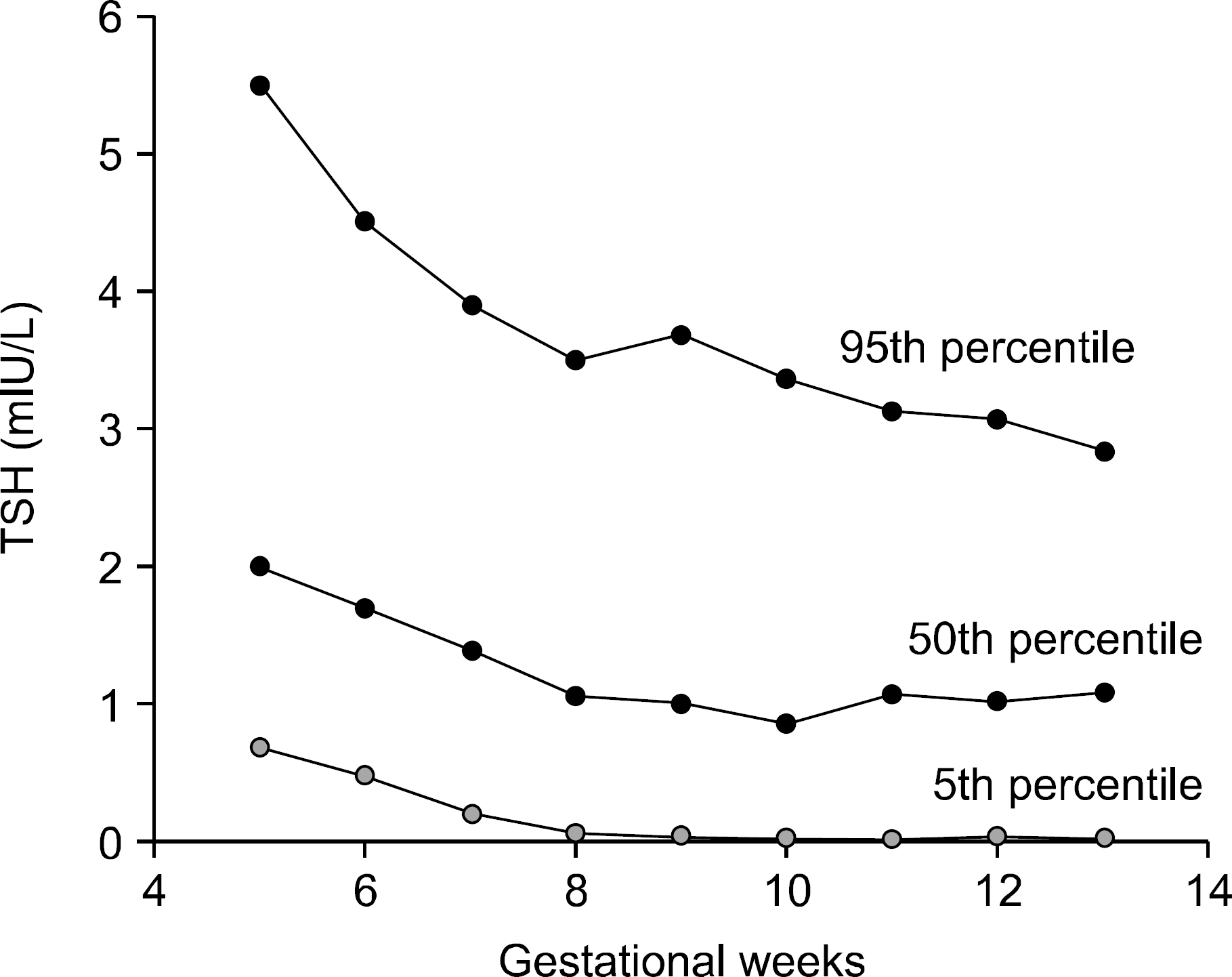
Table 1.
Percentile values of TSH of non-pregnant and each gestational age pregnant women
| Gestational weeks | Number | TSH (mIU/L) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2.5th | 5th | 25th | Median | 75th | 95th | 97.5th | p value∗ | p value† | ||
| Non-pregnant Pregnant (week) | 1835 | 0.31 | 0.59 | 1.26 | 1.87 | 2.73 | 4.73 | 5.70 | ||
| 5 | 247 | 0.52 | 0.69 | 1.33 | 2.00 | 3.00 | 5.50 | 6.05 | 0.061 | |
| 6 | 793 | 0.34 | 0.48 | 1.11 | 1.70 | 2.59 | 4.50 | 5.58 | <0.001 | <0.001 |
| 7 | 1384 | 0.08 | 0.20 | 0.77 | 1.40 | 2.17 | 3.91 | 4.73 | <0.001 | <0.001 |
| 8 | 1762 | 0.03 | 0.06 | 0.49 | 1.05 | 1.81 | 3.50 | 4.50 | <0.001 | <0.001 |
| 9 | 1156 | 0.02 | 0.03 | 0.44 | 0.99 | 1.72 | 3.68 | 4.38 | 0.058 | <0.001 |
| 10 | 374 | 0.01 | 0.02 | 0.35 | 0.87 | 1.61 | 3.36 | 3.90 | 0.083 | <0.001 |
| 11 | 563 | 0.00 | 0.02 | 0.43 | 1.07 | 1.73 | 3.13 | 3.52 | 0.139 | <0.001 |
| 12 | 1841 | 0.02 | 0.04 | 0.49 | 1.03 | 1.69 | 3.06 | 3.57 | 0.731 | <0.001 |
| 13 | 245 | 0.00 | 0.02 | 0.59 | 1.08 | 1.80 | 2.84 | 3.24 | 0.303 | <0.001 |
Table 2.
Prevalence of hypothyroidism with different diagnostic criteria




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download