Abstract
Anaplastic transformation of differentiated thyroid cancer at distant metastatic sites is extremely rare and has a poor prognosis. It usually occurs in the thyroid gland or cervical lymph nodes. Here we report a case of anaplastic transformation arising at multiple distant metastatic sites including the lung, liver, adrenal gland, bone, and lymph nodes in a patient 3 years after total thyroidectomy for follicular thyroid cancer.
Anaplastic thyroid carcinoma (ATC) is a rare but highly lethal form of thyroid cancer.1) ATC can arise de novo or, more commonly, through anaplastic transformation (or dedifferentiation) of preexisting differentiated thyroid cancer (DTC), including papillary thyroid carcinoma (PTC) or follicular thyroid carcinoma (FTC). Anaplastic transformation primarily occurs in the thyroid gland or in the cervical lymph nodes and less often in distant metastatic sites.23456)
Here we report a very rare case of anaplastic transformation arising at multiple distant metastatic sites including the lung, liver, adrenal gland, bone, and lymph nodes in a patient 3 years after total thyroidectomy for FTC.
A 72-year-old man was admitted on July 2015 with aggravated cough and sputum lasting 1 month, which had been unresponsive to medical treatment in a local clinic. He also had left-sided chest discomfort, fatigue, and poor oral intake. He underwent total thyroidectomy with central neck dissection due to a growing bulky thyroid mass on April 2012. Macroscopic examination revealed a huge, partly encapsulated but overtly infiltrative solid tumor, which occupied almost the entire left lobe and isthmus. The tumor measured approximately 7.5 cm at its widest point. The cut surface of the tumor was fleshy, firm, and tan white with a large area of cystic infarct. Microscopic examination revealed a variety of architectural patterns from area to area, including normofollicular, microfollicular, trabecular, and insular patterns (Fig. 1A). No papillary structures were noted. The majority of the tumor cells was cuboidal and had round to ovoid nuclei with relatively fine chromatin and inconspicuous nucleoli (Fig. 1B). Mitotic figures were infrequently noted. The tumor contained a minor component showing more serious atypia such as an insular pattern, cleaved nuclei, and occasional mitotic figures (Fig. 1C). The tumor revealed widespread extension to the thyroid parenchyma, perithyroidal soft tissue, and subcutaneous soft tissue. Multiple foci of vascular invasion were also present (Fig. 1A). No lymph node metastasis was noted. A diagnosis of widely invasive follicular carcinoma was made. He has been taking a daily dose of 0.15 mg of levothyroxine since the surgery and calcitriol and calcium carbonate for postoperative hypoparathyroidism. A radioactive iodine (RAI) ablation (150 mCi [5.5 GBq] of 131I) was administered 4 months after the surgery (August 2012). A post-therapeutic whole-body scan (RxWBS) revealed no uptake at the neck but functioning metastases to the sternum and both lungs (Fig. 2A), consistent with the findings of positron emission tomography-computed tomography (PET-CT), which was performed before the surgery (Fig. 2B). A stimulated serum thyroglobulin level was 53,980 ng/mL with a thyrotropin (TSH) level 51.71 mIU/L (0.25-4.0) with negative anti-thyroglobulin antibody. He had then been treated with sternum excision for sternum metastasis on August 2013 and two additional rounds of RAI therapy for multiple lung metastasis on April 2013 (150 mCi of 131I) and January 2014 (200 mCi of 131I). The last RxWBS showed significantly decreased uptake in the sternum and both lungs (Fig. 2A), and his stimulated serum thyroglobulin level decreased to 14,170 ng/mL. Thereafter, his unstimulated serum thyroglobulin level increased from 3,786 ng/mL to 12,380 ng/mL 3 months and 1 year after the third RAI therapy, respectively (Fig. 3). We recommended additional RAI therapy, but he was reluctant to undergo it. Meanwhile, he complained of pain at the surgical site of the sternum from bone cement bulging. The sternum was reconstructed on May 2015 and the surgical biopsy of excised soft tissue revealed only dense fibrosis with no evidence of malignancy. A chest X-ray showed no apparent metastatic nodules in the lung, and he did not complain of any symptoms. His condition was good enough to travel with family until 1 month before admission.
On admission, laboratory investigations were within normal limits, but his unstimulated serum thyroglobulin level increased to 23,330 ng/mL. A chest X-ray showed a new large mass in the retrocardiac area of the left lower lung zone and several small nodular opacities in both lungs. The chest CT revealed a new heterogeneous enhancing irregular mass, approximately 6.4 cm in size abutting the mediastinal pleura in the left lower lobe suggesting metastasis or primary lung cancer and increased size and number of multiple small and tiny nodules in both lungs suggesting hematogenous metastases compared with previous CT. Additionally, newly enlarged lymph nodes in the left supraclavicular area and a few heterogeneous enhanced masses in the sternal resection site of the upper anterior chest wall appeared, all suggesting metastases. Abdominal CT also showed two new low-density, heterogeneous enhanced masses in the liver and heterogeneous enhanced masses in left adrenal gland, approximately 1.2 cm and 2.8 cm in size, suggesting metastases. PET-CT showed a large, hypermetabolic lesion in the left lower lung and multiple hypermetabolic lesions in the liver, left adrenal gland, multiple whole-body skeletal areas including the sternum; multiple lymph nodes along the anterior mediastinum, left hilar, retrocaval, and left common iliac areas; and both lung fields (Fig. 2B). Fibrous tissue or necrotic endoluminal mass lesion was observed in the left lower posterior basal segmental bronchus by bronchoscopy, suspicious of bronchogenic carcinoma. Bronchoscopic washing and biopsy showed only necrotic cells and extensive necrosis, respectively. CTand fluoroscopy-guided percutaneous core needle biopsy was done for the left lower lung mass. The lung biopsy specimen showed diffuse infiltrate of anaplastic cells with single or multiple pleomorphic nuclei and macronucleoli (Fig. 4). Extensive coagulation necrosis and exuberant acute inflammation were present. There was a minor component showing well-differentiated follicular cells arranged in a normofollicular pattern. Immunohistochemically, pan-keratin (AE1/AE3) and galectin-3 were expressed in both the anaplastic cells and the well differentiated follicular cells; however, thyroid transcription factor-1 (TTF-1) and thyroglobulin were negative in the anaplastic cells and positive in the well-differentiated follicular cells. The diagnosis of metastatic anaplastic carcinoma of thyroid origin was made. He was only treated with antibiotics for combined pneumonia and antitussives and analgesics for cough and left-sided chest pain. However, his general condition deteriorated soon after, and he ultimately died from respiratory failure 1 month after admission.
ATC is an aggressive form of thyroid cancer with a poor prognosis; median survival time is only 5-6 months, and 1-year survival is approximately 20%. It accounts for 1.7% of all thyroid cancers.1) Although the pathogenesis of ATC remains unclear, anaplastic transformation from preexisting DTC has become a well-documented occurrence.25) In many cases (20-90%), patients have a history of a previously resected DTC or an ATC with a coexisting DTC after a histological examination.125) In addition, transitional zones between the differentiated and undifferentiated components or preserved immunohistochemical staining for thyroglobulin, TTF-1, and paired box protein Pax-8 (PAX-8) in staining in well-differentiated areas suggests the anaplastic transformation of preexisting DTC.15) The most common coexisting DTC is a well-differentiated PTC (often the tall cell variant) followed by the conventional or oncocytic (Hürthle cell) type FTC.125) Although anaplastic transformation of DTC most commonly occurs in the thyroid gland and regional lymph nodes,23456) previous studies have reported limited cases of occurrence at distant metastatic sites, including the pelvis,3) retroperitoneum,47) lung,56891011) mandible,12) submandibular space,13) liver,14) breast,15) and shoulder.16) These cases reported anaplastic transformation from PTC at distant metastatic sites with three exceptions. These three cases (including our patient) were from FTC.37) In these cases, development of anaplastic transformation ranged from 4 to 30 years after the primary diagnosis of DTC.345, 6, 78910111213141516) Therefore, it should be noted that anaplastic transformation can occur in metastatic sites even after long-term follow-up.5) Autopsy studies have demonstrated that ATC is often widely metastatic at the time of death, with two or more metastatic sites found in the vast majority of cases (84%). The most common metastatic sites are the lungs (78%), followed by the intrathoracic lymph nodes (58%), neck lymph nodes (51%), pleura (29%), adrenal glands (24%), liver (20%), and brain (18%).41017) Poor prognostic factors in patients with ATC include the presence of acute symptoms such as a tumor size >5 cm, distant metastases, and leukocytosis in addition to male gender, age >60 years, and the presence of extrathyroidal involvement.134)
Advances in molecular technologies have aided the understanding of the molecular pathogenesis of poorly differentiated thyroid cancer (PDTC) and ATC by suggesting a stepwise tumoral progression. This progression begins with well-differentiated cells, which turn into poorly differentiated cells, and finally, the development of ATCs.1819) While BRAF and RAS mutations are the main drivers of aggressive thyroid carcinoma, ATCs have a greater mutational burden. This burden includes a higher frequency of mutations in TP53, the TERT promoter, the PI3K/AKT/mTOR pathway effectors, SWI/SNF subunits, and histone methyltransferases compared with PDTCs. ATC is also characterized by genomic complexity and profound undifferentiation.1819) In addition, RI therapy has been associated with an increase in the probability of anaplastic transformation of DTC.51420) Sera et al.20) examined 32 DTC patients with distant metastasis and followed up for 10 years or until death. The authors reported that ineffective 131I therapy to show insufficient accumulation of 131I might trigger early anaplastic changes via a p53 gene mutation with poor prognosis. Postoperative 131I therapy was performed in 11 of 15 cases including ours.34567810121416) However, further studies are needed as it remains unclear whether 131I therapy is associated with the pathogenesis of anaplastic transformation.5)
In conclusion, we report a case of anaplastic transformation of FTC in multiple distant metastatic sites including the lung, liver, adrenal gland, multiple skeletal areas, and lymph nodes over a very short time period during simultaneous treatment for FTC which demonstrated a relatively stable clinical course. Although this event is extremely rare, clinicians should be aware of the possibility of anaplastic transformation of DTC in distant metastatic sites. Active surveillance and longterm follow-up are imperative in high-risk patients because DTC is characterized by exceptionally rapid progression with a dire prognosis and drastically affects the treatment modality and prognosis.
Figures and Tables
Fig. 1
Microscopic examination of the thyroid tumor. (A) Low-power view exhibiting normofollicular (right side) and microfollicular (left side) growth patterns. Widespread infiltration of the thyroid parenchyma and multifocal vascular invasions are present (H&E staining, original magnification ×40). (B) High-power view of follicular pattern with round nuclei and fine chromatin (H&E staining, original magnification ×400). (C) High-power view of insular pattern with cleaved nuclei and mitotic activity (H&E staining, original magnification ×400).

Fig. 2
Follow-up of images during the clinical course. (A) Post-therapeutic whole body scan (RxWBS). The first RxWBS revealed no uptake at the neck but functioning metastases to the sternum and both lungs (left). The second RxWBS showed much improved metastases in both lungs compared with the first RxWBS (center). The third RxWBS showed significantly decreased uptake in the sternum (right). (B) Positron emission tomography-computed tomography (PET-CT) scan. The initial PET-CT revealed hypermetabolic lesions in the left thyroid lobe, sternal manubrium, and both lungs. The second PET-CT scan showed decreased extent and numeric changes of pulmonary nodules and no change in the sternum. The third PET-CT showed significantly decreased metabolic and extent change in the sternum. The last PET-CT scan on admission showed multiple hypermetabolic lesions in the lung; liver; left adrenal gland; along the whole body skeletal system; in and around the skin area in front of the sternum; and in the lymph nodes along the anterior mediastinal, left hilar, retrocaval, and left common iliac areas.
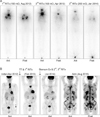
Fig. 3
Follow-up of thyroglobulin levels during the clinical course. Thyroglobulin levels were greatly decreased after two surgeries and three rounds of RITx. However, thereafter, thyroglobulin levels continuously increased throughout the course. Ex: excision, RAITx: radioactive iodine therapy, TSH: thyroid stimulating hormone, TT: total thyroidectomy

Fig. 4
Microscopic examination of the lung biopsy specimen. (A) Low-power view exhibiting diffuse infiltrate of anaplastic cells associated with extensive necrosis and acute inflammation (right side). There is a small focus of well-formed follicular architecture composed of well-differentiated follicular cells (left side) (H&E staining, original magnification ×100). (B) Higher magnification of anaplastic cells showing pleomorphic nuclei and macronucleoli (H&E staining, original magnification ×400).

References
1. Smallridge RC, Ain KB, Asa SL, Bible KC, Brierley JD, Burman KD, et al. American Thyroid Association guidelines for management of patients with anaplastic thyroid cancer. Thyroid. 2012; 22(11):1104–1139.

2. Wiseman SM, Loree TR, Rigual NR, Hicks WL Jr, Douglas WG, Anderson GR, et al. Anaplastic transformation of thyroid cancer: review of clinical, pathologic, and molecular evidence provides new insights into disease biology and future therapy. Head Neck. 2003; 25(8):662–670.

3. Nakayama R, Horiuchi K, Susa M, Hosaka S, Hayashi Y, Kameyama K, et al. Anaplastic transformation of follicular thyroid carcinoma in a metastatic skeletal lesion presenting with paraneoplastic leukocytosis. Thyroid. 2012; 22(2):200–204.

4. Solomon JP, Wen F, Jih LJ. Anaplastic transformation of papillary thyroid cancer in the retroperitoneum. Case Rep Pathol. 2015; 2015:241308.

5. Abe T, Suzuki M, Shimizu K, Shinagawa N, Oizumi S, Matsuno Y, et al. Anaplastic transformation of papillary thyroid carcinoma in multiple lung metastases presenting with a malignant pleural effusion: a case report. J Med Case Rep. 2014; 8:460.

6. Al-Qsous W, Miller ID. Anaplastic transformation in lung metastases of differentiated papillary thyroid carcinoma: an autopsy case report and review of the literature. Ann Diagn Pathol. 2010; 14(1):41–43.

7. Sotome K, Onishi T, Hirano A, Nakamaru M, Furukawa A, Miyazaki H, et al. A rare case of anaplastic transformation within the metastatic site of the retroperitoneal region in a patient 17 years after total thyroidectomy for papillary carcinoma of the thyroid beginning with multiple bone metastases. Thyroid. 2007; 17(12):1309–1311.

8. Moore JH Jr, Bacharach B, Choi HY. Anaplastic transformation of metastatic follicular carcinoma of the thyroid. J Surg Oncol. 1985; 29(4):216–221.

9. Khairy G. Anaplastic transformation of differentiated thyroid carcinoma. Int J Health Sci (Qassim). 2009; 3(1):93–96.
10. Benedict M, Costa J. Metastatic papillary thyroid carcinoma with multifocal synchronous transformation to anaplastic thyroid carcinoma. Case Rep Pathol. 2016; 2016:4863405.

11. Kim JS, Moon HJ, Han JS, Kim MJ. Importance of regular follow-up examination during active surveillance: a case of anaplastic transformation of papillary thyroid microcarcinoma. Int J Thyroidol. 2016; 9(2):185–189.

12. Ambelil M, Sultana S, Roy S, Gonzalez MM. Anaplastic transformation in mandibular metastases of follicular variant of papillary thyroid carcinoma: a case report and review of the literature. Ann Clin Lab Sci. 2016; 46(5):552–556.
13. Sumida T, Hamakawa H, Imaoka M, Okamoto N, Takarada M, Tanioka H, et al. A case of submandibular malignant rhabdoid tumor transformed from papillary thyroid carcinoma. J Oral Pathol Med. 2001; 30(7):443–447.

14. Takeshita Y, Takamura T, Minato H, Misu H, Ando H, Yamashita T, et al. Transformation of p53-positive papillary thyroid carcinoma to anaplastic carcinoma of the liver following postoperative radioactive iodine-131 therapy. Intern Med. 2008; 47(19):1709–1712.

15. Angeles-Angeles A, Chable-Montero F, Martinez-Benitez B, Albores-Saavedra J. Unusual metastases of papillary thyroid carcinoma: report of 2 cases. Ann Diagn Pathol. 2009; 13(3):189–196.

16. Kaushal S, Sharma MC, Mathur SR, Rastogi S, Bal CS, Chumber S. Anaplastic transformation of metastatic papillary thyroid carcinoma at shoulder mimicking soft tissue sarcoma. Indian J Pathol Microbiol. 2011; 54(4):796–799.
17. Besic N, Gazic B. Sites of metastases of anaplastic thyroid carcinoma: autopsy findings in 45 cases from a single institution. Thyroid. 2013; 23(6):709–713.

18. Xu B, Ghossein R. Genomic landscape of poorly differentiated and anaplastic thyroid carcinoma. Endocr Pathol. 2016; 27(3):205–212.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download