초록
Primary sclerosing mucoepidermoid carcinoma with eosinophilia (SMECE) of the thyroid gland is a very rare disease. We present the clinical and histopathologic findings of a 37-year-old woman recently diagnosed with SMECE of the thyroid gland. The patient, clinically euthyroid, who presented with a neck swelling since last 2 years along. Fine needle aspiration cytology suggested thyroid papillary carcinoma. Total thyroidectomy, central neck dissection and right selective neck dissection were performed. Although SMECE is considered to be a relatively slow growing and non-aggressive tumor, occasional metastasis does occur. We report an additional case of SMECE, with metastasis to regional lymph nodes. Physicians should be aware of extended operation, including total thyroidectomy and/or neck node dissection for metastatic lesion of the neck node. More standardized treatment is likely to evolve in the future.
REFERENCES
1). Chan JK, Albores-Saavedra J, Battifora H, Carcangiu ML, Rosai J. Sclerosing mucoepidermoid thyroid carcinoma with eosinophilia. A distinctive low-grade malignancy arising from the metaplastic follicles of Hashimoto's thyroiditis. Am J Surg Pathol. 1991; 15(5):438–48.
2). Rhatigan RM, Roque JL, Bucher RL. Mucoepidermoid carcinoma of the thyroid gland. Cancer. 1977; 39(1):210–4.

3). Geisinger KR, Steffee CH, McGee RS, Woodruff RD, Buss DH. The cytomorphologic features of sclerosing mucoepidermoid carcinoma of the thyroid gland with eosinophilia. Am J Clin Pathol. 1998; 109(3):294–301.

4). Sim SJ, Ro JY, Ordonez NG, Cleary KR, Ayala AG. Sclerosing mucoepidermoid carcinoma with eosinophilia of the thyroid: report of two patients, one with distant metastasis, and review of the literature. Hum Pathol. 1997; 28(9):1091–6.

5). Tomita T, Lotuaco L, Talbott L, Watanabe I. Mucoepidermoid carcinoma of the subglottis. An ultrastructural study. Arch Pathol Lab Med. 1977; 101(3):145–8.
6). Kay S. Mucoepidermoid carcinoma of the esophagus. Report of two cases. Cancer. 1968; 22(5):1053–9.

7). Hastrup N, Sehested M. High-grade mucoepidermoid carcinoma of the breast. Histopathology. 1985; 9(8):887–92.

8). Green LK, Gallion TL, Gyorkey F. Peripheral mucoepidermoid tumour of the lung. Thorax. 1991; 46(1):65–6.

9). Ohtsuki Y, Yoshino T, Takahashi K, Sonobe H, Kohno K, Akagi T. Electron microscopic study of mucoepidermoid carcinoma in the pancreas. Acta Pathol Jpn. 1987; 37(7):1175–82.

10). Diaz-Perez R, Quiroz H, Nishiyama RH. Primary mucinous adenocarcinoma of thyroid gland. Cancer. 1976; 38(3):1323–5.

11). Franssila KO, Harach HR, Wasenius VM. Mucoepidermoid carcinoma of the thyroid. Histopathology. 1984; 8(5):847–60.

12). Katoh R, Sugai T, Ono S, Takayama K, Tomichi N, Kurihara H, et al. Mucoepidermoid carcinoma of the thyroid gland. Cancer. 1990; 65(9):2020–7.

13). Steele SR, Royer M, Brown TA, Porter C, Azarow KS. Mucoepidermoid carcinoma of the thyroid gland: a case report and suggested surgical approach. Am Surg. 2001; 67(10):979–83.
14). Wenig BM, Adair CF, Heffess CS. Primary mucoepidermoid carcinoma of the thyroid gland: a report of six cases and a review of the literature of a follicular epithelial-derived tumor. Hum Pathol. 1995; 26(10):1099–108.

15). Chung J, Lee SK, Gong G, Kang DY, Park JH, Kim SB, et al. Sclerosing mucoepidermoid carcinoma with eosinophilia of the thyroid glands: a case report with clinical manifestation of recurrent neck mass. J Korean Med Sci. 1999; 14(3):338–41.

16). Solomon AC, Baloch ZW, Salhany KE, Mandel S, Weber RS, LiVolsi VA. Thyroid sclerosing mucoepidermoid carcinoma with eosinophilia: mimic of Hodgkin disease in nodal metastases. Arch Pathol Lab Med. 2000; 124(3):446–9.
17). Baloch ZW, Solomon AC, LiVolsi VA. Primary mucoepidermoid carcinoma and sclerosing mucoepidermoid carcinoma with eosinophilia of the thyroid gland: a report of nine cases. Mod Pathol. 2000; 13(7):802–7.

18). Bondeson L, Bondeson AG, Thompson NW. Papillary carcinoma of the thyroid with mucoepidermoid features. Am J Clin Pathol. 1991; 95(2):175–9.

19). Shehadeh NJ, Vernick J, Lonardo F, Madan SK, Jacobs JR, Yoo GH, et al. Sclerosing mucoepidermoid carcinoma with eosinophilia of the thyroid: a case report and review of the literature. Am J Otolaryngol. 2004; 25(1):48–53.

20). Kim JH, Kim SM, Hong SW, Chang HS, Park JS. Sclerosing mucoepidermoid carcinoma with eosinophilia of the thyroid: a case report with distant metastasis. Korean J Endocr Surg. 2014; 14(4):243–6.

21). Cavazza A, Toschi E, Valcavi R, Piana S, Scotti R, Carlinfante G, et al. Sclerosing mucoepidermoid carcinoma with eosinophilia of the thyroid: description of a case. Pathologica. 1999; 91(1):31–5.
22). Sharma K, Nigam S, Khurana N, Chaturvedi KU. Sclerosing mucoepidermoid carcinoma with eosinophilia of the thyroid–a case report. Indian J Pathol Microbiol. 2003; 46(4):660–1.
23). Hunt JL, LiVolsi VA, Barnes EL. p63 expression in sclerosing mucoepidermoid carcinomas with eosinophilia arising in the thyroid. Mod Pathol. 2004; 17(5):526–9.

24). Kanat Ö, Evrensel T, Tolunay Ş, Demìray M, Gönüllü G, Kurt E, et al. Sclerosing mucoepidermoid carcinoma with eosinophilia of the thyroid gland: description of a case and review of the literature. Turk J Cancer. 2004; 34(3):122–6.
Fig. 1.
Neck computed tomography with contrast enhance-ment demonstrating tumor invasion of the right thyroid lobe. A right supraclavicular lymph node metastasis is present (arrow).
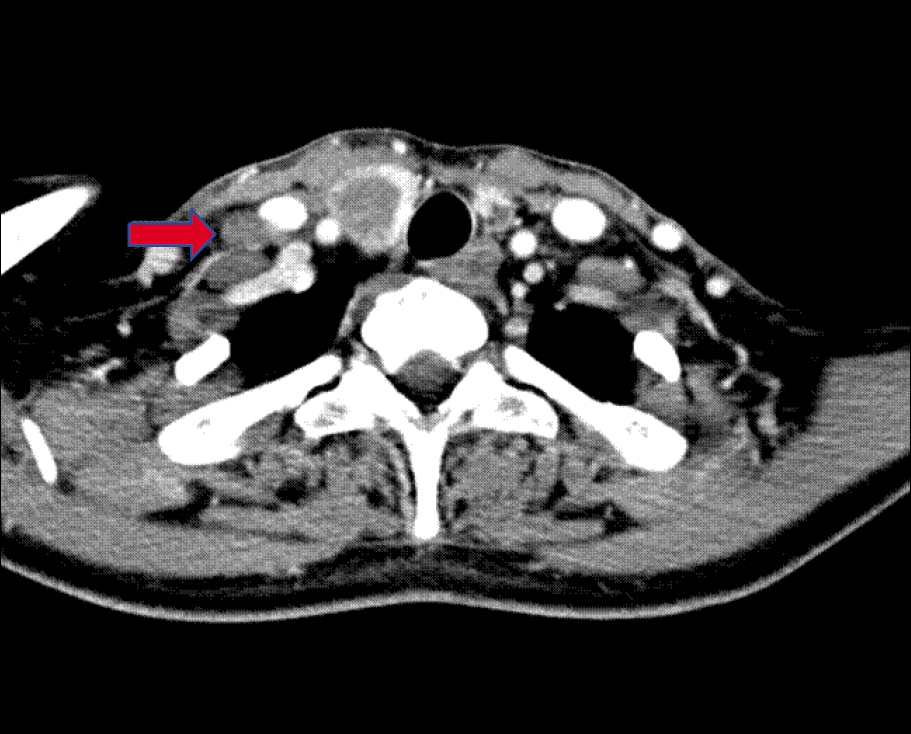
Fig. 2.
18F-fludeoxyglucose (FDG) positron emission tomography (PET) scan shows a metastatic lymphadenopathy at right supraclavicular area (arrow).
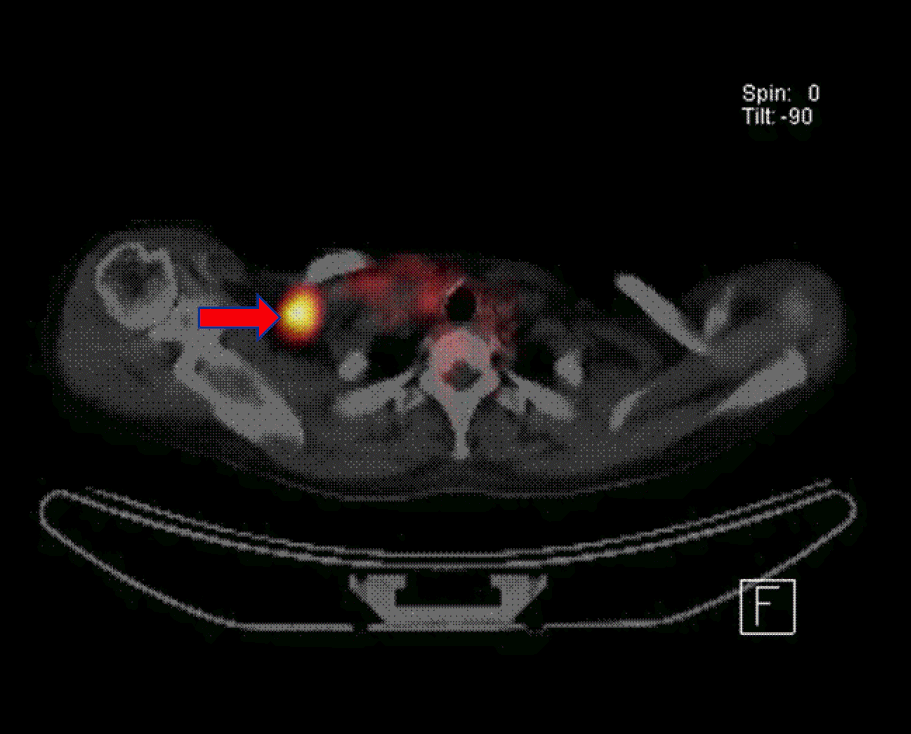
Fig. 3.
The tumor nodule has ill-defined border and adjacent thyroid reveals lymphocytic thyroiditis (H&E staining, ×40).
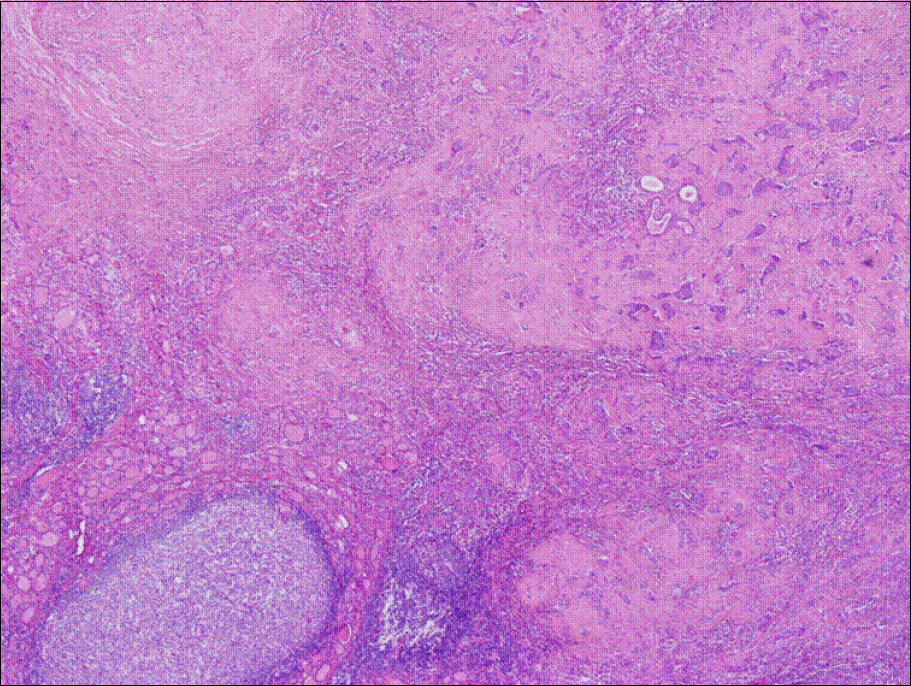
Fig. 4.
The tumor is characterized by anastomosing compact cords and nests of epidermoid cells, accompanied by extensive sclerosis and by infiltration of eosinophils, lymphocytes and plasma cells (A: H&E staining, ×100; B: inlet, ×400).
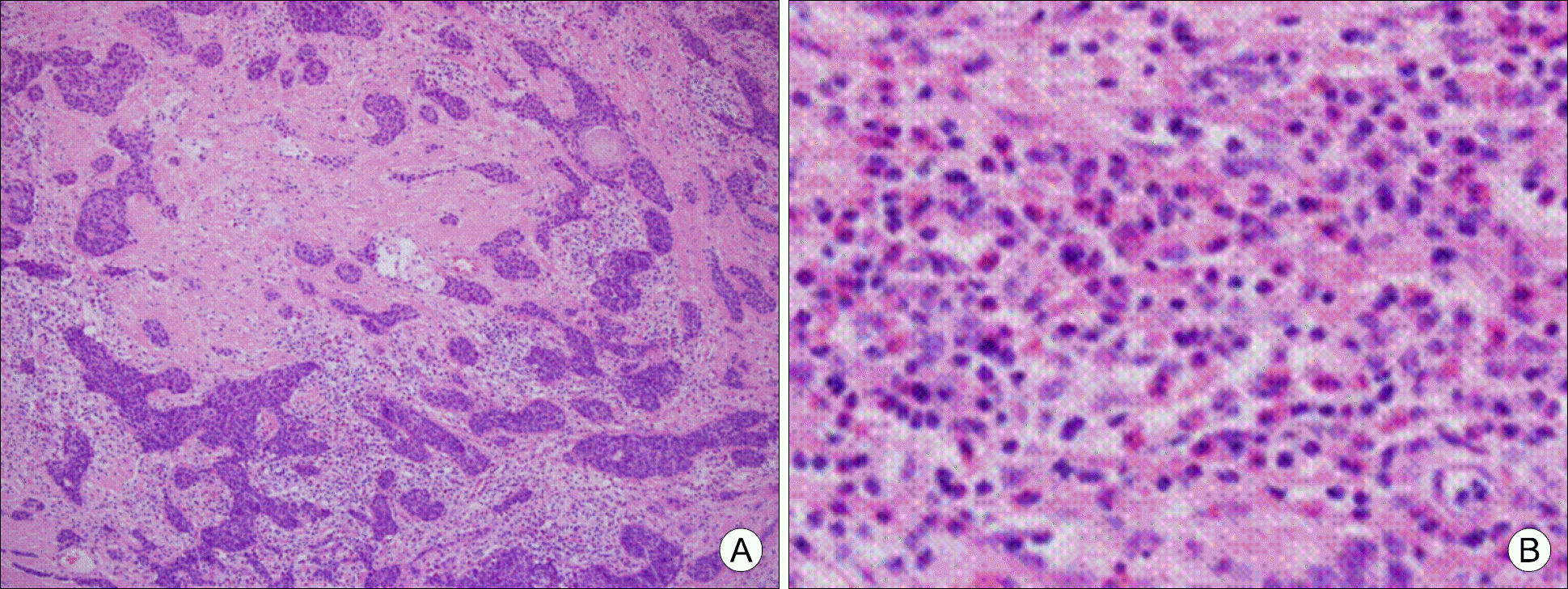
Fig. 5.
(A) Tumor contains a well-formed squamous pearl. (B) Mucocytes compressed by globules of mucin is interspersed in epidermoid cells (H&E staining, ×400).
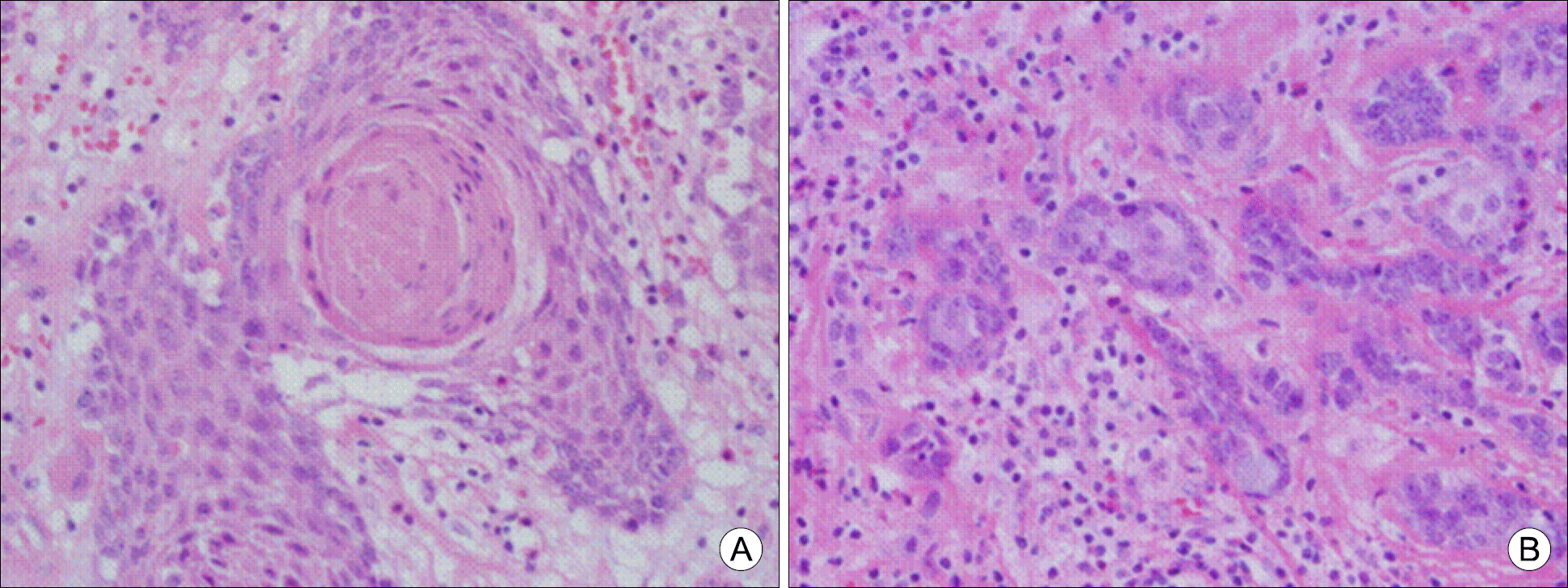
Table 1.
Literature review of sclerosing mucoepidermoid carcinoma with eosinophilia of the thyroid gland




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download