Introduction
Methods
Ethics
Subjects
Sample Size
Study Design
Plasma Aceclofenac Assay
Pharmacokinetics Analysis
Safety Assessment
Statistical Analysis
Results
Study Participants
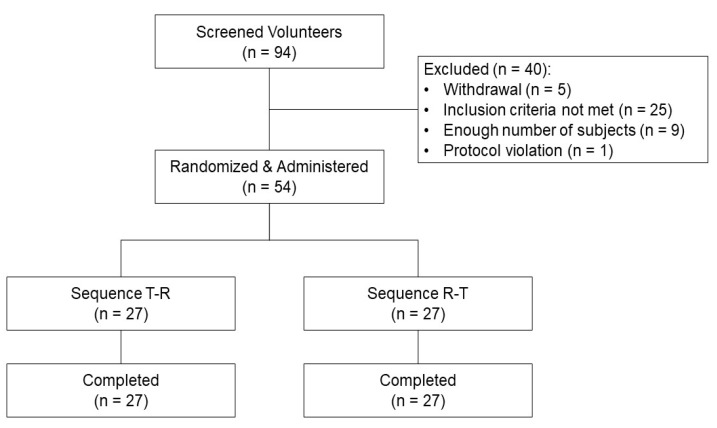 | Figure 1Subject disposition. Abbreviations: Sequence T–R, a fixed-dose combination tablet of SKI306X 300 mg /aceclofeanc 100 mg once (treatment T) was administered first in period 1, then co-administration of 300 mg of SKI306X and 100 mg of aceclofenac as individual tablets once (treatment R) in period 2; Sequence R–T, treatment R first in period 1, then treatment T in period 2. |
Table 1
Demographics and baseline characteristics of the study population
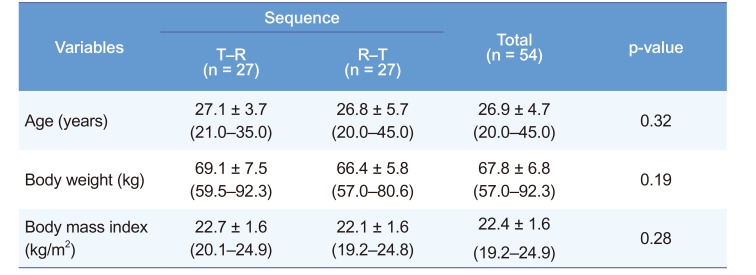
Notes: The data are expressed as the mean ± SD (min–max). Abbreviations: Sequence T–R, a fixed-dose combination tablet of SKI306X 300 mg /aceclofenac 100 mg once (treatment T) was administered first in period 1, then co-administration of 300 mg of SKI306X and 100 mg of aceclofenac as individual tablets once (treatment R) in period 2; Sequence R–T, treatment R first in period 1, then treatment T in period 2. The p-values between the two groups in each part were calculated using the Wilcoxon rank sum test. There were no statistically significant differences in age, body weight, and body mass index.
Pharmacokinetics Parameters
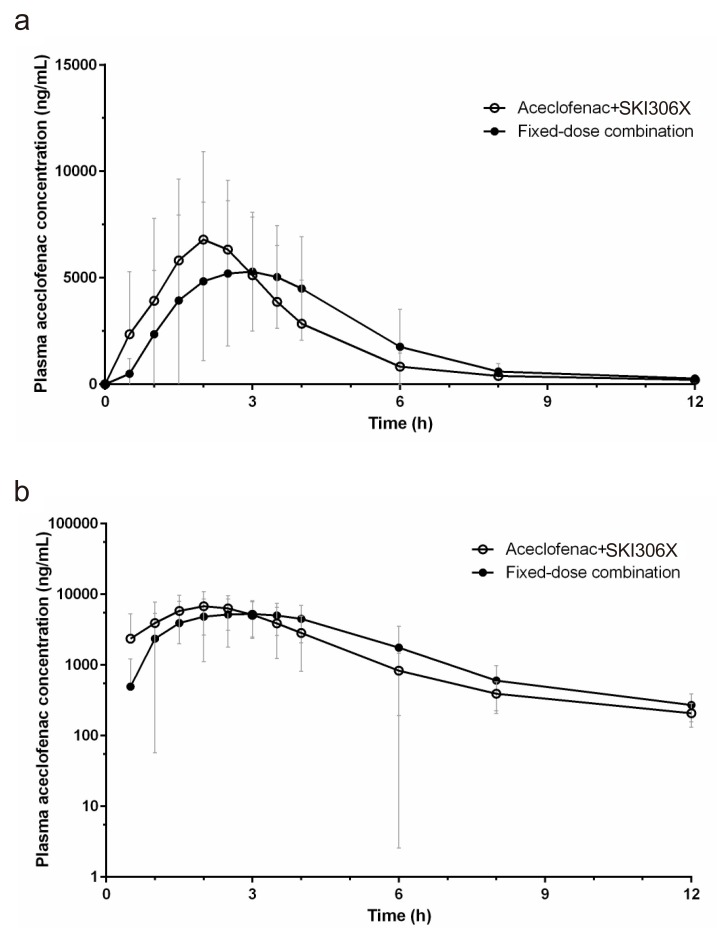 | Figure 2Mean (SD) plasma concentration-time profiles of aceclofenac (a: linear scale, b: semi-logarithmic scale). |
Table 2
Pharmacokinetic parameters of aceclofenac after the administration of a fixed-dose combination tablet that included 300 mg of SKI306X and 100 mg of aceclofenac once (treatment T) and co-administration of a SKI306X 300 mg and an aceclofenac 100 mg once (treatment R) in healthy volunteers
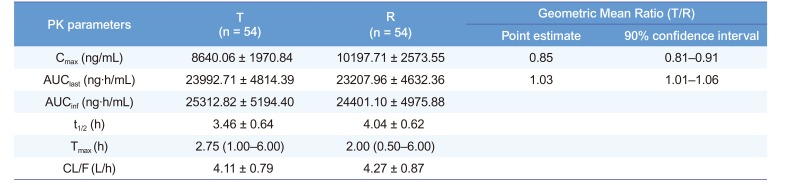
Notes: The values are presented as the mean ± SD, except for the tmax, which is presented as the median (range). Abbreviations: PK, pharmacokinetic; Cmax, maximum plasma concentration of the drug; AUClast, area under the plasma concentration-time curve from the dosing time to the last measurable concentration; AUCinf, AUC from the dosing time extrapolated to infinity; t1/2, elimination half-life; Tmax, time to Cmax; CL/F, apparent total clearance.
Safety
Table 3
Summary of adverse events
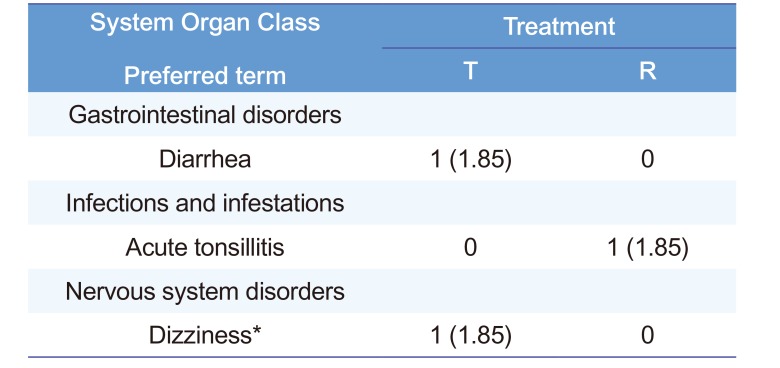
| System Organ Class Preferred term | Treatment | |
|---|---|---|
| T | R | |
| Gastrointestinal disorders | ||
| Diarrhea | 1 (1.85) | 0 |
| Infections and infestations | ||
| Acute tonsillitis | 0 | 1 (1.85) |
| Nervous system disorders | ||
| Dizziness* | 1 (1.85) | 0 |
Notes: The data are expressed as the number (%) of adverse events. *Dizziness is considered as drug-related AE. Abbreviations: T, treatment group administered a fixed-dose combination tablet that included 300 mg of SKI306X and 100 mg of aceclofenac once; R, treatment group administered 300 mg of SKI306X and 100 mg of aceclofenac concomitantly once.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download