Abstract
A lumbar puncture can be used to measure the concentrations of drugs and/or pharmacodynamic biomarkers during clinical trials of central nervous system drugs. We report a case of a post lumbar puncture headache (PLPH) in a first-in-human study, which was reported as a serious adverse event. A 20-year-old man received 200 mg of the investigational product (IP) for 7 days and underwent a lumbar puncture for cerebrospinal fluid sampling before IP administration (Day 1, pre-dose) and after 7 days and multiple IP administrations (Day 7, 1 hour post-dose). After discharge on Day 8, the subject complained of headache, nausea, vomiting, neck stiffness, and numbness of the extremities. The symptoms occurred when he got up and disappeared after he remained in the supine position for several minutes. Five days later, he visited the neurology clinic of the main hospital. The neurologist recommended hospitalization for further evaluation and symptom management, and the subject was then admitted to the hospital. There were no abnormal findings in vital signs, laboratory results, or brain-computed tomography. His symptoms disappeared during the hospitalization period. It was important to distinguish whether the headache was IP-related or lumbar puncture-related. Therefore, knowledge of clinical characteristics and differential diagnosis of PLPH is paramount. Furthermore, if severe PLPH occurs, a consultation with a neurologist and imaging studies should be considered for a differential diagnosis of PLPH.
A lumbar puncture is usually performed for various purposes. It can be used for regional anesthesia, or to assist in making a differential diagnosis of a potential life-threatening disease, including bacterial meningitis and subarachnoid hemorrhage.[1] Because the drug concentration in the cerebrospinal fluid (CSF) is often used as an important marker of central nervous system (CNS) penetration, lumbar puncture is widely used in early phase clinical trials of CNS drugs.[2] Post lumbar puncture headache (PLPH) can occur at a frequency of 32% in a clinical setting.[3] According to the Headache Classification Committee of the International Headache Society, PLPH is characterized as a “headache [that] has developed within 5 days after lumbar puncture and remits spontaneously within 2 weeks. It is usually accompanied by neck stiffness and/or subjective hearing symptoms”.[4] Serious complications of lumbar puncture, including meningitis, epidural hematoma, subdural hematoma, and nerve palsy, are rare, but they must be evaluated.[5] We report a case of PLPH in a healthy subject participating in the first-in-human study of an anti-Parkinson drug. This case was also reported to the Institutional Review Board (IRB) of CHA Bundang Medical Center as a serious adverse event (SAE).
A 20-year-old man presented with a severe headache. He was a subject in the first-in-human study at CHA hospital Clinical Trials Center (CTC). The IRB of CHA Bundang Medical Center approved the study protocol (IRB number: 2016-07-067-019), and this study was registered in a public trial registry (ClinicalTrials.gov, registration number: NCT03022799). The subject received 200 mg of KM-819, the investigational product (IP) orally for 7 days. He underwent a lumbar puncture for CSF sampling before IP administration (Day 1, pre-dose) and after 7 days and multiple IP administrations (Day 7, 1 hour post-dose). A neurologist performed the lumbar puncture using a traumatic 22G needle. The subject was discharged on the morning of Day 8, and started to complain of a severe throbbing headache of grade 3 according to the Common Terminology Criteria for Adverse Events (CTCAE, version 4), occurring at the bilateral frontal areas, one hour after discharge. In addition, he experienced nausea, vomiting, neck stiffness, and numbness of the extremities (CTCAE grades 1 or 2). These symptoms occurred when he got up and disappeared after he was supine for several minutes. We diagnosed the condition as PLPH because he tried to remain in the “supine position” to avoid aggravation of headache, which is a characteristic that is more likely to be related to the spinal tap than to the IP. Five days later, he visited CTC for a follow-up examination. His headache had aggravated, so we referred him to the neurology clinic for further evaluation and management. The neurologist recommended hospitalization for symptom management, and the subject was then admitted to the hospital.
The subject did not show any abnormal neurological findings during a neurologic examination at the neurology clinic. There were no abnormal findings in his vital signs, laboratory results, or brain-computed tomography (CT) (Fig. 1).
After admission, the subject was kept in a supine position and was hydrated and treated with acetaminophen for his headache. The headache improved to be mild in severity, and other symp-toms disappeared. The subject was discharged on the third day of hospitalization, and his headache was completely resolved without neurological sequelae two days after discharge.
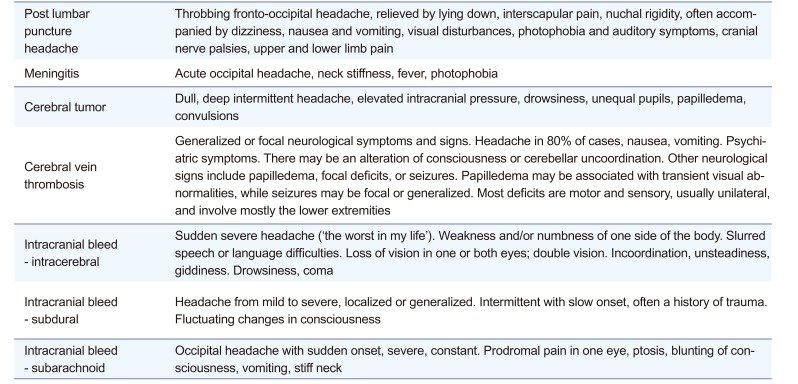
According to the most recent European Medicines Agency guideline, if at least one subject experienced an SAE that could be considered to be at least possibly related to an IP administration, the investigator should stop dosing and terminate the clinical trial.[6] Furthermore, any serious unexpected suspected adverse reaction (SUSAR) should be reported to the regulatory authorities and independent ethics committees (IEC)/IRB no later than 15 days post the occurrence of the adverse reaction. Therefore, it was important to distinguish whether the headache was IP-related or lumbar puncture-related. PLPH is usually diagnosed on the basis of clinical features. The key points that distinguish PLPH are that it generally appears or worsens within 15 minutes of an upright postural change and characteristically subsides within 30 minutes of recumbence.[37] The differential diagnosis of PLPH is broadly ranged (Table 1).[89] Therefore, since many potentially life-threatening conditions, such as subarachnoid hematoma and cerebral venous thrombosis, may seem to be PLPH, knowledge about the clinical characteristics and differential diagnosis of PLPH is paramount. The criteria for differential diagnosis include severity, onset, characteristics, association with posture, level of consciousness, and other accompanying symptoms, such as neck stiffness, fever, visual disturbance, photophobia, weakness, ptosis, cranial nerve palsy, and seizures. Typical features of potentially life-threatening diseases are summarized in Table 2.[10] According to the subject's symptoms, the headache was much closer to a PLPH than it was to other diseases.
Administration of the IP can also cause headaches. KM-819 (KR33493) is a fatty acid synthase-associated factor 1 (FAF1) inhibitor.[11] A FAF1 can suppress nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) activity through cytoplasmic retention of NF-κB p65.[1213] NF-κB activation might be associated with provocation of migraine attacks at the neuronal level.[1415] NF-κB seems to mediate the transcriptional signal to inducible nitric oxide synthase and inflammatory cytokines, and can be activated by various pathological and inflammatory stimuli, such as oxidative stress.[1617] Therefore, administration of the IP might provoke migraine attacks by activating NF-κB. Actually, the subject did complain of a moderate throbbing headache of the right occipital areas before the second lumbar puncture, during the confinement period at the CTC. However, unlike with a PLPH, this first headache was not alleviated by a supine position, and there were no complaints of neck stiffness or back pain. Therefore, this headache might have been associated with the IP and also have disappeared after acetaminophen treatment.
We had thorough discussions during an interim safety meeting to determine any causal relationship between the SAE and the IP with sponsor representatives and a medical monitor of the contract research organization. The clinical features of both a PLPH and a migraine are throbbing with accompanied nausea, vomiting, neck stiffness, and numbness. Therefore, it was difficult to distinguish a PLPH from a migraine. However, the locations of a PLPH are usually bilateral rather than unilateral, as in a migraine.[31819] Because the most important feature of a PLPH is aggravation of the headache when in an upright position, we ruled out migraines and simple headaches for the present case. We also ruled out the possibility of potentially life-threatening diseases due to lack of abnormal findings in the brain CT, a rapid recovery by only conservative treatments, and the neurologist's opinion that the subject's symptoms had likely been caused by the lumbar puncture. Therefore, we concluded that the SAE was unlikely to be related to the IP. Hence, the SAE would neither require reporting as a SUSAR nor lead to a halt to the next dose group. In addition, we did not unblind the subject, because it was irrelevant for him to know whether he was on active or placebo treatment for his severe headache.
PLPH can also occur after a lumbar puncture in healthy volunteers. Because of the pressure gradient between the intradural and extradural spaces, CSF leaks into the epidural space through the hole on the dura mater. The CSF pressure gradient in young adults is higher than that in elderly, so CSF loss and the risk of a PLPH is more common in young adults.[20] According to one prospective study, the risk of a PLPH was highest in 20 to 30-year-old people. This age group was 3–5 times more likely to develop a PLPH than those aged greater than 60 years. [21] Considering that most volunteers of first-in-human studies are in this high-risk age group, investigators should pay attention to the risk of the occurrence of a PLPH when performing a lumbar puncture. Risk factors for a PLPH are large needle size, needle design, replacement of the stylet, and number of lumbar puncture attempts.[3] In clinical practice, a 22G needle is the best for lumbar puncture, because thinner needles require a much longer time for CSF collection.[22] To reduce the incidence of PLPH, an expert physician should perform a lumbar puncture using an atraumatic needle.[2324] Nevertheless, if severe PLPH occurs, neurologist consultation and imaging studies, such as CT or magnetic resonance imaging, should be considered for a differential diagnosis of PLPH.
This case report has some limitations. First, although the subject experienced neck stiffness and numbness of the extremities, he did not undergo a spinal magnetic resonance imaging scan. Therefore, we cannot completely exclude the possibility of an epidural abnormality. Second, there is no specific guideline for determining the relationship between the IP and a headache occurring after lumbar puncture in early-phase clinical trials of CNS drugs. To establish such a guideline, a large number of PLPH cases in several studies should be analyzed. If a guideline is to be established, it will be helpful in differentiating the cause of the headaches. Although these limitations exist, this case report is worth discussing when using lumbar punctures during early-phase clinical trials involving healthy subjects.
Acknowledgements
This study was registered on Clinicaltrials.gov (NCT03022799) and was supported by Kainos Medicine, Inc., Seongnam, Gyeonggi-do, 13488, Republic of Korea.
Notes
References
1. Doherty CM, Forbes RB. Diagnostic lumbar puncture. Ulster Med J. 2014; 83:93–102. PMID: 25075138.
2. Shen DD, Artru AA, Adkison KK. Principles and applicability of CSF sampling for the assessment of CNS drug delivery and pharmacodynamics. Adv Drug Deliv Rev. 2004; 56:1825–1857. PMID: 15381336.

3. Ahmed SV, Jayawarna C, Jude E. Post lumbar puncture headache: diagnosis and management. Postgrad Med J. 2006; 82:713–716. PMID: 17099089.

4. Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013; 33:629–808. PMID: 23771276.
5. Amini A, Liu JK, Kan P, Brockmeyer DL. Cerebrospinal fluid dissecting into spinal epidural space after lumbar puncture causing cauda equina zsyndrome: review of literature and illustrative case. Childs Nerv Syst. 2006; 22:1639–1641. PMID: 16933137.
6. European Medicines Agency. Guideline on strategies to identify and mitigate risks for first-in-human and early clinical trials with investigational medicinal products (20 Jul 2017). Accessed 10 August 2017. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2017/07/WC500232186.pdf.
7. Lavi R, Rowe JM, Avivi I. Lumbar puncture: it is time to change the needle. Eur Neurol. 2010; 64:108–113. DOI: 10.1159/000316774. PMID: 20628255.

8. Bezov D, Lipton RB, Ashina S. Post-dural puncture headache: part I diagnosis, epidemiology, etiology, and pathophysiology. Headache. 2010; 50:1144–1152. DOI: 10.1111/j.1526-4610.2010.01699.x. PMID: 20533959.

9. Gielen M. Post dural puncture headache (PDPH): a review. Reg Anesth. 1989; 14:101–106. PMID: 2486588.
10. Bleeker CP, Hendriks IM, Booij LH. Postpartum post-dural puncture head ache: is your differential diagnosis complete? Br J Anaesth. 2004; 93:461–464. PMID: 15220177.
11. Jeong JW, Yu C, Lee JH, Moon KS, Kim E, Yoo SE, et al. Subacute toxicity evaluation of KR-33493, FAF1 inhibitor for a new anti-parkinson's disease agent, after oral administration in rats and dogs. Regul Toxicol Pharmacol. 2016; 81:387–396. DOI: 10.1016/j.yrtph.2016.09.022. PMID: 27664323.

12. Menges CW, Altomare DA, Testa JR. FAS-associated factor 1 (FAF1): diverse functions and implications for oncogenesis. Cell Cycle. 2009; 8:2528–2534. PMID: 19597341.

13. Park MY, Jang HD, Lee SY, Lee KJ, Kim E. Fas-associated factor-1 inhibits nuclear factor-kappaB (NF-kappaB) activity by interfering with nuclear translocation of the RelA (p65) subunit of NF-kappaB. J Biol Chem. 2004; 279:2544–2549. PMID: 14600157.
14. Greco R, Tassorelli C, Cappelletti D, Sandrini G, Nappi G. Activation of the transcription factor NF-kappaB in the nucleus trigeminalis caudalis in an animal model of migraine. Neurotoxicology. 2005; 26:795–800. PMID: 15936821.
15. Reuter U, Chiarugi A, Bolay H, Moskowitz MA. Nuclear factor-kappaB as a molecular target for migraine therapy. Ann Neurol. 2002; 51:507–516. PMID: 11921057.

16. Coppola G. Neurophysiology of Migraine. In : Ashina M, Geppetti P, editors. Pathophysiology of headaches: From Molecule to Man. Switzerland: Springer International Publishing;2015. p. 155–174.
17. Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009; 1:a001651. DOI: 10.1101/cshperspect.a001651. PMID: 20457564.
18. Bruera OC, Bonamico L, Giglio JA, Sinay V, Leston JA, Figuerola ML. Intracranial hypotension: the nonspecific nature of MRI findings. Headache. 2000; 40:848–852. PMID: 11135032.

19. Solomon S, Lipton RB. Criteria for the diagnosis of migraine in clinical practice. Headache. 1991; 31:384–387. PMID: 1889979.

20. Fleischman D, Berdahl JP, Zaydlarova J, Stinnett S, Fautsch MP, Allingham RR. Cerebrospinal fluid pressure decreases with older age. PLoS One. 2012; 7:e52664. DOI: 10.1371/journal.pone.0052664. PMID: 23300737.

21. Lybecker H, Moller JT, May O, Nielsen HK. Incidence and prediction of postdural puncture headache. A prospective study of 1021 spinal anesthesias. Anesth Analg. 1990; 70:389–394. PMID: 2316881.

22. Carson D, Serpell M. Choosing the best needle for diagnostic lumbar puncture. Neurology. 1996; 47:33–37. PMID: 8710120.

23. Lavi R, Yarnitsky D, Rowe JM, Weissman A, Segal D, Avivi I. Standard vs atraumatic Whitacre needle for diagnostic lumbar puncture: a randomized trial. Neurology. 2006; 67:1492–1494. PMID: 17060584.

24. Ross BK, Chadwick HS, Mancuso JJ, Benedetti C. Sprotte needle for obstetric anesthesia: decreased incidence of post dural puncture headache. Reg Anesth. 1992; 17:29–33. PMID: 1599891.
Table 1
Differential diagnosis of headache after lumbar puncture
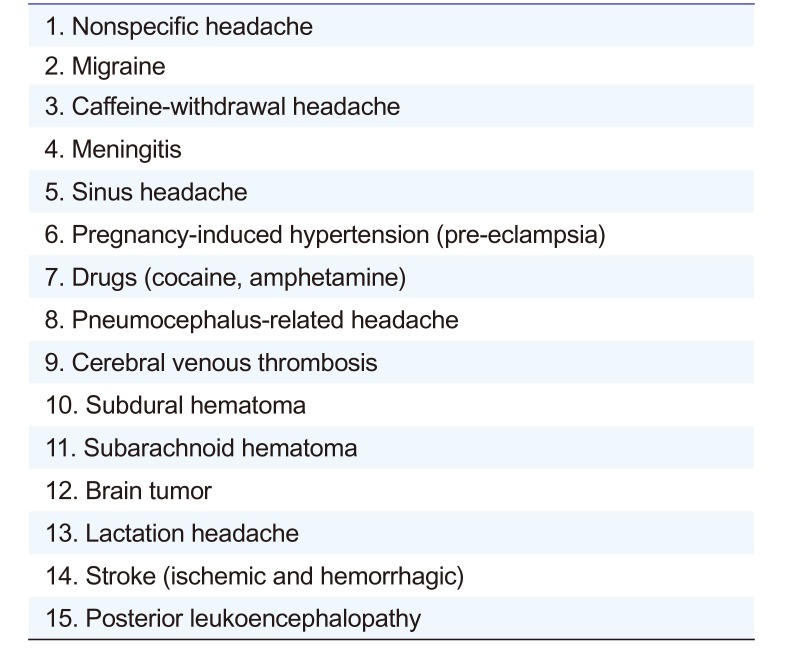
Table 2
Typical features of post lumbar puncture headache and potential life-threatening diseases after lumbar puncture[10]




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download