1. Ingelman-Sundberg M, Oscarson M, McLellan RA. Polymorphic human cytochrome P450 enzymes: An opportunity for individualized drug treatment. Trends Pharmacol Sci. 1999; 20:342–349. PMID:
10431214.

2. Budnitz DS, Shehab N, Kegler SR, Richards CL. Medication use leading to emergency department visits for adverse drug events in older adults. Ann Intern Med. 2007; 147:755–765. PMID:
18056659.

3. Ma MK, Woo MH, McLeod HL. Genetic basis of drug metabolism. Am J Health Syst Pharm. 2002; 59:2061–2069. PMID:
12434718.

4. Nebert DW, Wikvall K, Miller WL. Human cytochromes P450 in health and disease. Philos Trans R Soc Lond B Biol Sci. 2013; 368:20120431. DOI:
10.1098/rstb.2012.0431. PMID:
23297354.

6. Ingelman-Sundberg M. Genetic polymorphisms of cytochrome P450 2D6 (CYP2D6): Clinical consequences, evolutionary aspects and functional diversity. Pharmacogenomics J. 2005; 5:6–13. PMID:
15492763.

7. Zanger UM, Schwab M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther. 2013; 138:103–141. DOI:
10.1016/j.pharmthera.2012.12.007. PMID:
23333322.

8. Sim SC, Ingelman-Sundberg M. Update on allele nomenclature for human cytochromes P450 and the human cytochrome P450 allele (CYP-allele) nomenclature database. Methods Mol Biol. 2013; 987:251–259. DOI:
10.1007/978-1-62703-321-3_21. PMID:
23475683.

9. Bernard S, Neville KA, Nguyen AT, Flockhart DA. Interethnic differences in genetic polymorphisms of CYP2D6 in the U.S. Population: Clinical implications. Oncologist. 2006; 11:126–135. PMID:
16476833.

10. Caudle KE, Dunnenberger HM, Freimuth RR, Peterson JF, Burlison JD, Whirl-Carrillo M, et al. Standardizing terms for clinical pharmacogenetic test results: Consensus terms from the Clinical Pharmacogenetics Implementation Consortium (CPIC). Genet Med. 2017; 19:215–223. DOI:
10.1038/gim.2016.87. PMID:
27441996.

11. Lee SJ, Lee SS, Jung HJ, Kim HS, Park SJ, Yeo CW, et al. Discovery of novel functional variants and extensive evaluation of CYP2D6 genetic polymorphisms in Koreans. Drug Metab Dispos. 2009; 37:1464–1470. DOI:
10.1124/dmd.108.022368. PMID:
19364831.
12. Kim EY, Lee SS, Jung HJ, Jung HE, Yeo CW, Shon JH, et al. Robust CYP2D6 genotype assay including copy number variation using multiplex single-base extension for Asian populations. Clin Chim Acta. 2010; 411:2043–2048. DOI:
10.1016/j.cca.2010.08.042. PMID:
20828547.

13. Gaedigk A, Gotschall RR, Forbes NS, Simon SD, Kearns GL, Leeder JS. Optimization of cytochrome P450 2D6 (CYP2D6) phenotype assignment using a genotyping algorithm based on allele frequency data. Pharmacogenetics. 1999; 9:669–682. PMID:
10634130.
14. Eichelbaum M, Woolhouse NM. Inter-ethnic difference in sparteine oxidation among Ghanaians and Germans. Eur J Clin Pharmacol. 1985; 28:79–83. PMID:
3987789.

15. Schmid B, Bircher J, Preisig R, Kupfer A. Polymorphic dextromethorphan metabolism: Co-segregation of oxidative O-demethylation with debrisoquin hydroxylation. Clin Pharmacol Ther. 1985; 38:618–624. PMID:
4064464.

16. Sachse C, Brockmöller J, Hildebrand M, Müller K, Roots I. Correctness of prediction of the CYP2D6 phenotype confirmed by genotyping 47 intermediate and poor metabolizers of debrisoquine. Pharmacogenetics. 1998; 8:181–185. PMID:
10022755.

17. Chou WH, Yan FX, Robbins-Weilert DK, Ryder TB, Liu WW, Perbost C, et al. Comparison of two CYP2D6 genotyping methods and assessment of genotype-phenotype relationships. Clin Chem. 2003; 49:542–551. PMID:
12651805.

18. Sabbagh A, Darlu P. Data-mining methods as useful tools for predicting individual drug response: Application to CYP2D6 data. Hum Hered. 2006; 62:119–134. PMID:
17057402.
19. Gaedigk A, Simon SD, Pearce RE, Bradford LD, Kennedy MJ, Leeder JS. The CYP2D6 activity score: Translating genotype information into a qualitative measure of phenotype. Clin Pharmacol Ther. 2008; 83:234–242. PMID:
17971818.

20. Hicks JK, Swen JJ, Gaedigk A. Challenges in CYP2D6 phenotype assignment from genotype data: A critical assessment and call for standardization. Curr Drug Metab. 2014; 15:218–232. PMID:
24524666.

21. Gaedigk A, Sangkuhl K, Whirl-Carrillo M, Klein T, Leeder JS. Prediction of CYP2D6 phenotype from genotype across world populations. Genet Med. 2017; 19:69–76. DOI:
10.1038/gim.2016.80. PMID:
27388693.

22. Streetman DS, Ellis RE, Nafziger AN, Leeder JS, Gaedigk A, Gotschall R, et al. Dose dependency of dextromethorphan for cytochrome P450 2D6 (CYP2D6) phenotyping. Clin Pharmacol Ther. 1999; 66:535–541. PMID:
10579482.

23. Droll K, Bruce-Mensah K, Otton SV, Gaedigk A, Sellers EM, Tyndale RF. Comparison of three CYP2D6 probe substrates and genotype in Ghanaians, Chinese and Caucasians. Pharmacogenetics. 1998; 8:325–333. PMID:
9731719.

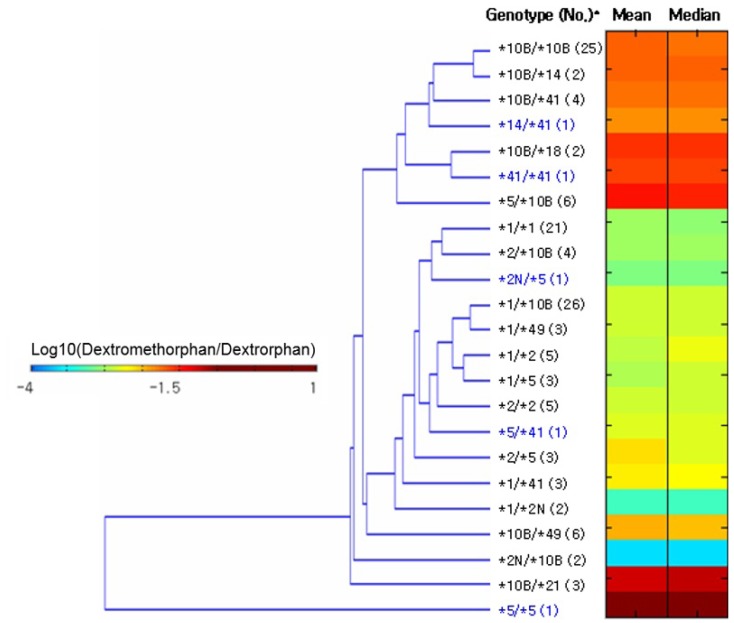
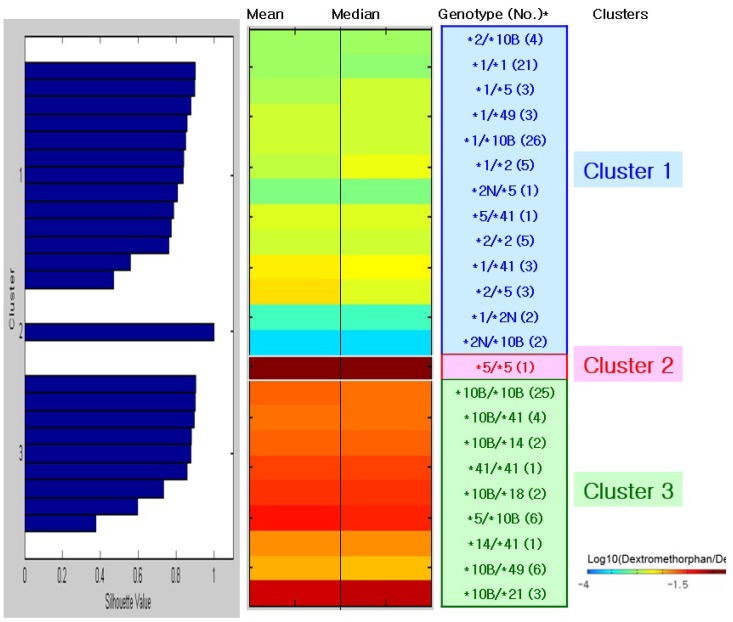
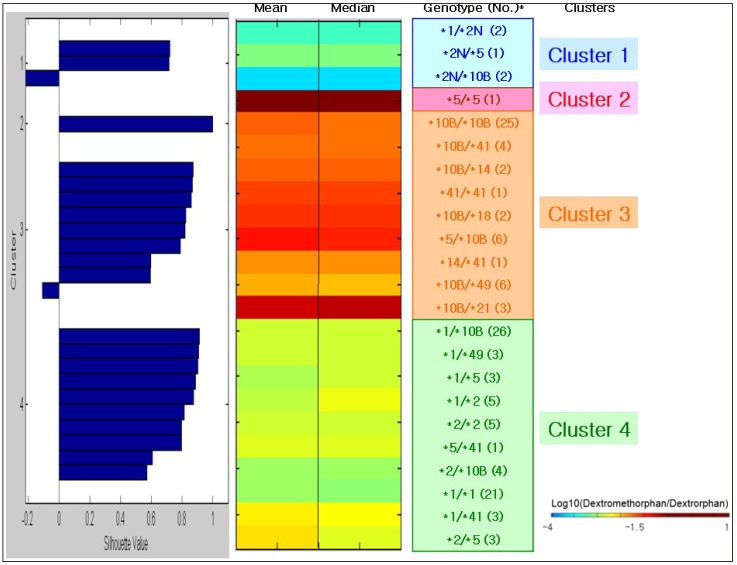
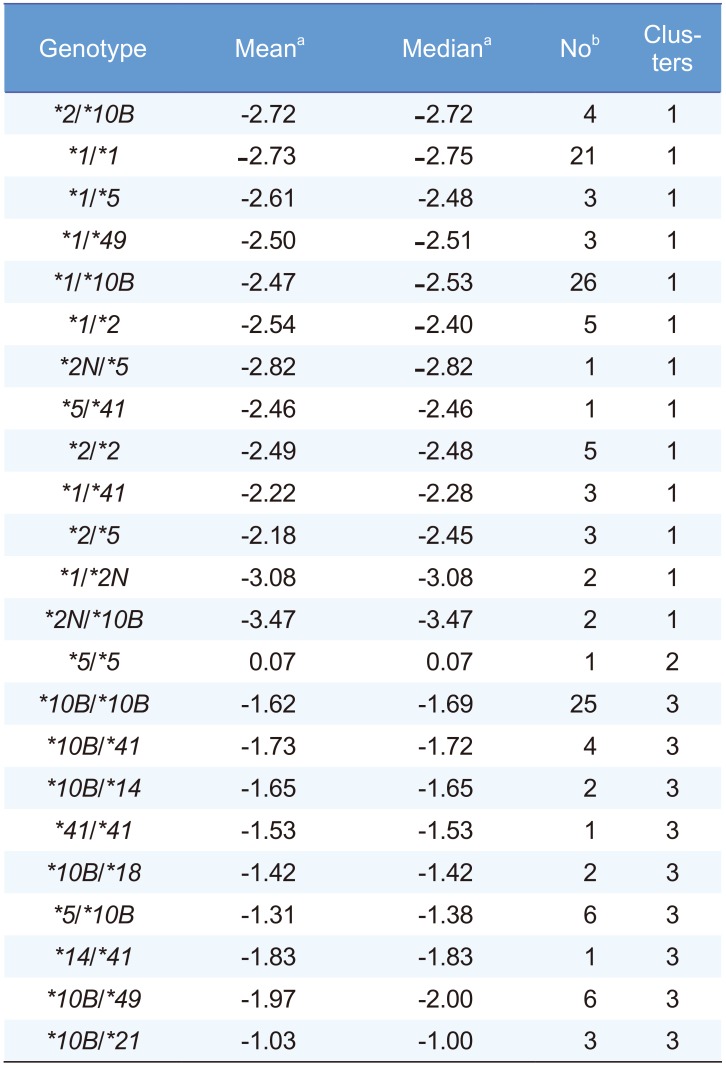




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download