Abstract
Rivaroxaban is a new oral anticoagulant used for the prevention of stroke in patients with atrial fibrillation. Hemorrhagic pericarditis is known to occur with rivaroxaban; however, only a few case reports in the literature describe such events. Recently, we experienced hemorrhagic pericarditis that treated with rivaroxaban for anticoagulation of newly diagnosed, non valvular AF patients with pacemaker. An 83 year old male with permanent pacemaker receiving rivaroxaban 20 mg daily once for 3 months presented at our emergency department complaining of exertional dyspnea. ECG showed intermittent atrial pacing failure and echocardiography showed large amount of pericardial effusion. After urgent pericardiocentesis, which resulted in removal of 500cc bloody fluid, there was an immediate and dramatic improvement in the patient's clinical state. He was discharged without anticoagulation therapy due to concern for further bleeding. This case highlight the potential for bleeding complications associated with novel anticoagulants. Rivaroxaban is being used with increasing frequently in outpatient care. However, no available laboratory test specifically measures the anticoagulant effect of rivaroxaban. Also, in the events of serious bleeding, no specific antidotes, reversal agents were available. Clinicians should be aware of the possibility of hemopericardium in patients treated with anticoagulants, including rivaroxaban who presented with cardiomegaly.
Atrial fibrillation (AF) is the most commonly encountered cardiac arrhythmia. Anticoagulation therapy must be needed to reduce the occurrence of systemic embolic events in high-risk patients. Rivaroxaban is a new oral anticoagulant used for the prevention of stroke in patients with AF.[1] Various bleeding complications with rivaroxaban have been reported.[2] Hemorrhagic pericarditis is known to occur with rivaroxaban; however, only a few case reports in the literature have described such events.[345] Recently, we experienced a case of hemorrhagic pericarditis in a patient treated with rivaroxaban for anticoagulation of newly diagnosed, nonvalvular AF.
An 83-year-old man presented at our emergency department complaining of exertional dyspnea and pleuritic chest pain. Upon physical examination, his blood pressure was 100/70 mmHg and his irregular heart rate was 64 beats per minute. On auscultation, his breathing sound was coarse. He had undergone percutaneous coronary intervention for unstable angina with implantation of a drug-eluting stent (DES; 3.5×28 mm Taxus stent; Boston Scientific, Boston, MA, USA) in the left anterior descending artery 10 years ago. Three months before presenting, a dual chamber permanent pacemaker was implanted for symptomatic tachy-brady syndrome (AF plus sinus pause). He had been prescribed clopidogrel 75 mg, rivaroxaban 20 mg, bisoprolol 2.5 mg, and rosuvastatin 20 mg once daily at the outpatient clinic for 3 months.
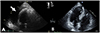
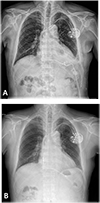
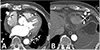
At the emergency department, electrocardiography (ECG) on presentation demonstrated intermittent atrial pacing failure (Fig. 1) Pacemaker interrogation showed the following parameter changes: pacing threshold, 0.5 V → 1.5 V at 40 ms; sensing threshold, 2.8 mV → 1.5 mV; and impedance, 430 → 410 ohms. Chest radiography revealed significant cardiomegaly and bilateral pleural effusions, which had not been demonstrated in previous chest radiography. The results of initial blood testing were as follows: white blood cell count, 14,200 mm3; hemoglobin (Hb), 11.7 g/dL; platelets, 155,000 mm3; prothrombin time, 32.5 s (international normalized ratio of 1.13); activated partial prothrombin time, 46.0 s; D-dimer levels, 6.04 mg/mL; fibrin degradation product levels, 12.8 mg/mL; and C-reactive protein, 10.3 mg/dL. His cardiac enzyme levels were within normal limits (CK-MB, 0.5 ng/mL; Troponin T, 0.16 ng/mL). The patient's serum creatinine was in the normal range (1.1 mg/dL) with an estimated creatinine urinary clearance of 53 mL/min (using the Cockcroft-Gault equation) 3 months ago. It was elevated at 1.9 mg/dL with an estimated creatinine clearance of 29.17 mL/min. Portable transthoracic echocardiography showed large amounts of pericardial effusion (Fig. 2) After urgent pericardiocentesis, which resulted in removal of 500 mL bloody fluid, there was an immediate and dramatic improvement in the patient's clinical state. Cytology of the pericardial fluid revealed no malignant cells. The fluid had a Hb level of 6.0 g/dL, whereas the patient's serum Hb level was 9.1 g/dL. Rivaroxaban was discontinued because of concern for hemopericardium and to allow for normalization of the drug-induced coagulopathy. A workup for other causes of the hemopericardium, including pacemaker lead dislodgement, malignancy, and autoimmune disease-related pericarditis was negative. Serologic tests for syphilis, hepatitis B, hepatitis C, and HIV were negative. Rheumatoid factor, antinuclear antibody, anti-dsDNA, anti-Sm, anti-RNP, anti-Ro, anti-La, and anti-neutrophil cytoplasmic antibodies were all negative. Chest radiography and computed tomography showed proper lead position and no aortic dissection (Figs. 3 and 4) On the seventh day, repeat transthoracic echocardiogram confirmed no further accumulation of pericardial effusion and the pigtail catheter was removed, having drained a total of 1000 mL of heavily blood-stained fluid. He was discharged with antiplatelet agents prescribed because of concern for further bleeding, and has been followed up at our outpatient department without further symptoms for 2 years.
Rivaroxaban is a novel anticoagulant approved for use in patients with AF for preventing stroke. However, uncontrolled bleeding with this drug is a considerable problem because there is no direct antidote or blood product to reverse its anticoagulant effects entirely. A 5.5% chance of major bleeding is associated with rivaroxaban use, including intracranial and gastrointestinal bleeding.[2] Few reports are available regarding hemorrhagic pericarditis associated with rivaroxaban use.[345] This was a case of isolated hemopericardium related to rivaroxaban use, which could potentially lead to death from pacing failure or cardiac tamponade.
There are several possible explanations for the development of hemorrhagic pericarditis associated with rivaroxaban in our patient. First, we suspected atrial lead dislodgement or perforation. The ECG showed intermittent pacing artifacts without capture (Fig. 1) However, atrial leads were in stable, appropriate positions as viewed on chest radiography or computed tomography scans. Pacemaker implantation seems to be linked to the formation of a hemopericardium, which might be caused by oozing because of perforation of the lead (screw type) and anticoagulants and antiplatelet drugs might exacerbate bleeding. Second, rivaroxaban at 20 mg was prescribed in this case. Rivaroxaban is typically dosed at this rate once daily, reducing to 15 mg once daily for patients with a GFR of 15–50 mL/min. Patients receiving rivaroxaban are exposed to increased bleeding risks secondary to rivaroxaban overdose in cases of acute kidney injury. All other cases with hemorrhagic pericarditis associated with rivaroxaban use were accompanied with transient renal dysfunction, as in our case.[345] Clinicians should be aware that dose reduction might be needed when the GFR is calculated to be low if serum creatinine is within the normal range for older patients, especially octogenarians.[6] Third, it is possible that combination therapy with antiplatelet agents might have increased the likelihood of bleeding. The combination of AF with coronary artery disease is a complex setting to treat with anticoagulation and antiplatelet therapy and it is associated with higher mortality rates. Especially, in such patients with DES implantation, appropriate anticoagulation therapy is very important to prevent stroke and stent thrombosis, but there is always a potential risk for serious hemorrhagic complications. There are insufficient data available to guide clinical practice optimally in such a setting. Current guidelines recommend antiplatelet agents and anticoagulation therapy for patients with AF after the deployment of a coronary artery stent for 1 year.[7] The combination of antiplatelet medication that could increase the risk of bleeding should be carefully reviewed for any patients on an anticoagulant. The workup for other causes of hemopericardium, including malignancy and autoimmune disease-related pericarditis, was negative in our case.
This case highlights the potential for bleeding complications associated with novel anticoagulants. Clinicians should be aware of the possibility of a hemopericardium in patients treated with anticoagulants, including rivaroxaban and should be particularly cautious in elderly patients, those with transient renal dysfunction, and those receiving antiplatelet agents that might affect serum drug concentrations.
Figures and Tables
Figure 2
Transthoracic ECG showed a large amount of pericardial effusion (A), which had not been demonstrated 3 months before (B).
References
1. Fox KA, Piccini JP, Wojdyla D, Becker RC, Halperin JL, Nessel CC, et al. Prevention of stroke and systemic embolism with rivaroxaban compared with warfarin in patients with non-valvular atrial fibrillation and moderate renal impairment. Eur Heart J. 2011; 32:2387–2394. DOI: 10.1093/eurheartj/ehr342.

2. Piccini JP, Garg J, Patel MR, Lokhnygina Y, Goodman SG, Becker RC, et al. Management of major bleeding events in patients treated with rivaroxaban vs. warfarin: results from the ROCKET AF trial. Eur Heart J. 2014; 35:1873–1880. DOI: 10.1093/eurheartj/ehu083.

3. Shivamurthy P, Brar N, Therrien ML. Isolated hemopericardium associated with rivaroxaban: first case report. Pharmacotherapy. 2014; 34:e169–e172. DOI: 10.1002/phar.1474.

4. Boone S. Cardiac tamponade associated with rivaroxaban. Del Med J. 2015; 87:206–207.
5. Xu B, MacIsaac A. Life-threatening haemorrhagic pericarditis associated with rivaroxaban. Int J Cardiol. 2014; 174:e75–e76. DOI: 10.1016/j.ijcard.2014.04.151.

6. Halperin JL, Hankey GJ, Wojdyla DM, Piccini JP, Lokhnygina Y, Patel MR, et al. Efficacy and safety of rivaroxaban compared with warfarin among elderly patients with nonvalvular atrial fibrillation in the Rivaroxaban Once Daily, Oral, Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF). Circulation. 2014; 130:138–146. DOI: 10.1161/CIRCULATIONAHA.113.005008.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download