Abstract
We report a case of Waldenström's macroglobulinemia (WM) treated using clarithromycin (CAM) and prednisolone (PSL). An 84-year-old woman was admitted to our hospital for bleeding after a tooth extraction and hematuria. Computed tomography showed multiple ill-defined nodules in the omentum (omental cake). Although the cause of the omental cake remained unclear, the patient was diagnosed with WM, based on the detection of M-protein of immunoglobulin (Ig) M in serum and lymphoplasmacytes in bone marrow. The bleeding tendency in the patient may have been due to acquired hemophilia and/or hyper IgM-induced platelet dysfunction. The patient was treated using CAM (800 mg/day) and PSL (10 mg/day). As a result, IgM levels gradually decreased. Because the omental cake contracted along with improvement in IgM, it was thought to be lymphoplasmacytic lymphoma-like lymphoma. This case shows that treatment using CAM and PSL may be effective in some cases of WM.
Macrolides have been considered as potential antineoplastic and immunomodulatory agents. As antineoplastic agents, macrolides show anti-lymphoproliferative effects.[1] Various lymphoid malignancies, such as mucosa-associated lymphoid tissue (MALT) lymphoma,[2] follicular B-cell lymphoma,[3] diffuse large B-cell lymphoma,[4] Waldenström's macroglobulinemia (WM),[5] multiple myeloma (MM),[6] and angioimmunoblastic T-cell lymphoma,[7] have been successfully treated using clarithromycin (CAM), a macrolide. Here, we present a patient with WM accompanied by lymphoma successfully treated using CAM and prednisolone (PSL).
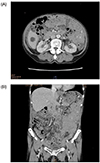
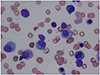
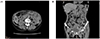
An 84-year-old woman was admitted to our hospital for bleeding after a tooth extraction and hematuria. Physical examination showed neither hepatosplenomegaly nor superficial lymphadenopathy. Petechiae were not found on her skin. A solid tumor, approximately 25 cm in longitudinal size, was palpable in her left abdomen. Laboratory findings were as follows: white blood cell count, 5,580/µL (neutrophils, 72.2%; eosinophils, 2.0%; monocytes, 4.3%; and lymphocytes, 21.3%); red blood cell count, 291 × 104/µL; hemoglobin, 8.0 g/dL; platelet count, 22.4 × 104/µL; total protein, 12.8 g/dL; albumin, 2.8 g/dL; and C-reactive protein, 0.66 mg/dL. Liver and renal function parameters were normal. Serological tests showed the following results: immunoglobulin (Ig) G, 731 mg/dL; IgM, 8,620 mg/dL (normal range, 35–122 mg/dL); and IgA, 120 mg/dL. M-protein of IgM-κ type was detected using immunoelectrophoresis. β2 microglobulin level was 6.7 mg/L (normal range, 0.9–1.9 mg/L). Anti-nuclear antibody titer was ×40. Anti-cardiolipin beta-2-glycoprotein I antibody level was <1.3 U/mL (normal value, <3.5 U/mL). Among the tumor markers, soluble interleukin-2 receptor (sIL-2R) level was 4,788 U/mL (normal range, 122–496 U/mL) and CA125 level was 397.5 U/mL (normal value, <35.0 U/mL). Other tumor markers, such as CEA and CA19-9, were within the normal limits. Prothrombin time was 14.2 s (normal range, 10.0–15.0 s), but activated partial thromboplastin time was prolonged (45.3 s; normal range, 26.0–38.0 s). Factor VIII level was 33.6 pg/mL (normal range, 78.0–165.0 pg/mL), whereas factor IX, X, and XI levels were within the normal limits. A bone marrow aspiration smear revealed a nucleated cell count of 121,000/µL comprising 6% lymphoplasmacytes (Fig. 1) and a megakaryocyte count of 78.1/µL. Cytogenetic analysis revealed no chromosomal abnormalities. Contrast-enhanced computed tomography (CT) showed slight hepatomegaly, multiple ill-defined nodules in the omentum (omental cake), an omental tumor accompanied by vessel augmentation, para-aortic lymphadenopathy, and ascites (Fig. 2A, B). It also showed no abnormalities in the lungs, gall bladder, pancreas, bilateral kidneys, and bladder, but revealed stenosis of the lower part of the left ureter. Cystoscope revealed bleeding from the left ureteral orifice. Gynecological examination revealed no abnormalities in the uterus or bilateral ovaries. The patient refused to undergo esophagogastroduodenoscopy and colonoscopy. Percutaneous CT- or ultrasound-guided biopsy of the omental cake was not permitted because of the fear of bleeding; therefore, the cause of the omental cake remained unclear. Finally, based on the detection of M-protein of IgM in serum and lymphoplasmacytes in bone marrow, the patient was diagnosed with WM. Although inhibitor assay to Factor VIII was not performed, the bleeding tendency in the patient may have been due to acquired hemophilia[8] and/or hyper IgM-induced platelet dysfunction.[9] The hematuria was thought to be due to the omental tumor invasion of the left ureter. Because the patient did not want to receive anticancer drugs for fear of adverse reactions, such as nausea, vomiting, and loss of hair, she was treated using hemostatic agents, such as tranexamic acid and carbazochrome sodium sulfonate hydrate, and then discharged. Two weeks after discharge, she experienced slight visual disturbance and abdominal discomfort, and her IgM level increased to 10,300 mg/dL. Therefore, we suggested plasma exchange and chemotherapy, but she refused these. Hence, we prescribed PSL (10 mg/day) and CAM (800 mg/day) on the basis of their anti-lymphoproliferative effects, after taking informed consent. Approximately 10 weeks after initiating this treatment, hematuria improved and visual disturbance did not worsen. CT findings showed that the size of the omental tumor had halved. In addition, IgM, and sIL-2R levels decreased to 6,205 mg/dL, and 931 U/mL, respectively, and Factor VIII level increased to 63.2 pg/mL. Approximately 5 months after initiating this treatment, the omental cake, the omental tumor, para-aortic lymphadenopathy, and ascites considerably improved (Fig. 3A, B). In addition, IgM and sIL-2R levels decreased to 3,680 mg/dL and 626 U/mL, respectively, and Factor VIII level increased to 132.2 pg/mL. Because the omental cake, the omental tumor, and para-aortic lymphadenopathy contracted along with improvement in IgM, these lesions were thought to be lymphoplasmacytic lymphoma-like lymphoma.
Metastatic involvement is the most common cause of omental cakes. Along with peritoneal fluid and peritoneal thickening with enhancement, omental involvement is frequently encountered with peritoneal carcinomatosis on CT. While ovarian carcinoma is the most common cause of omental cakes, colonic, pancreatic, and gastric cancers are other common malignancies that may result in omental metastasis. Primary malignancies and benign tumors of the peritoneum and omentum, such as abdominal mesothelioma, hemangiopericytoma, leiomyosarcoma, lipoma, liposarcoma, neurofibroma, fibrosarcoma, and gastrointestinal stromal and small round cell tumors, are rare. Therefore, these tumors should be considered in the absence of a known or suspected primary organ-based malignancy.[10] The omentum comprises a fibrous tissue structure without lymphoid tissues; therefore, non-Hodgkin lymphoma with omental involvement is rare. At present, the mechanisms underlying lymphoma invasion into omental pathways remain unclear, but have been hypothesized to be similar to gastrointestinal cancer metastasis of cancer cells spreading through the transverse mesocolon and stomach mesocolone surface.[11] Qi et al. reported 14 cases with omental cake-like thickening in the autopsy of 322 non-Hodgkin lymphoma cases.[11] From these facts, we initially speculated that the omental cake and tumor may have been due to metastatic involvement other than hematological malignancy. However, clinical course proved that they were related to WM. Therefore, the possibility of omental involvement of lymphomas should be considered.
CAM has recently been considered to have anti-proliferative activities against lymphoid malignancies.[1] Ishimatsu et al. reported two cases of pulmonary MALT lymphoma successfully treated using CAM.[2] They speculated that macrolide induced the apoptosis of lymphocytes via downregulation of bcl-xL, an anti-apoptotic protein.[12] We have also previously reported a case of follicular B-cell and diffuse large B-cell lymphomas successfully treated using CAM.[34] CAM is known to induce the downregulation of bcl-2, an anti-apoptotic protein,[13] and lymphoma cells from our previous cases were positive for bcl-2, so we speculated that CAM induced the apoptosis of lymphoma cells. Coleman et al. reported the combination of CAM with low-dose thalidomide, and dexamethasone in patients with WM resulted in an 83% response rate.[5] They speculated that CAM induced apoptosis directly or altered corticosteroid metabolism.[5] Niesvizky et al. reported the combination of CAM with lenalidomide and dexamethasone in patients with MM resulted in a 90.3% response rate.[6] They speculated that the addition of CAM to dexamethasone enhanced the glucocorticoid effect. Recently, it was reported that CAM augmented the anti-tumor activity of thalidomide against MM cells via the attenuation of autophagy of these cells, eventually leading to cell death.[14] As for T-cell lymphoma, we have previously reported a case of angioimmunoblastic T-cell lymphoma successfully treated using CAM.[7] We speculated that CAM targeted the tumor microenvironment by inhibiting the vascular endothelial growth factor. Glucocorticoid (GC) is also known to induce the apoptosis of lymphoid cells. In fact, GC-induced apoptosis has been exploited in the therapy of lymphoid malignancies. [15] Considering the apoptosis of lymphoid cells, CAM and PSL treatment may be beneficial in the treatment of lymphoproliferative diseases. Biopsy specimens from the omental cake were not obtained in this case, so it was unclear whether anti-apoptotic proteins were present. However, in this case, based on the fact that omental involvement considerably improved when treated using CAM and PSL, anti-lymphoproliferative effects including those mentioned above were thought to operate. Because CAM and PSL treatment in this case indicated 64.2% reduction of IgM levels, our patient achieved partial response according to the previous report.[5] Although our patient appears to have benefited from CAM and PSL treatment, this treatment cannot be recommended at present. Our case demonstrated that this treatment can be considered as an option in patients refusing chemotherapy or in elderly patients with underlying medical conditions.
More extensive research is required to substantiate our findings before this treatment can be adopted on a wider basis.
Figures and Tables
Figure 2
(A) Contrast-enhanced computed tomography (CT) images showing para-aortic lymphadenopathy, multiple ill-defined nodules in the omentum (omental cake), an omental tumor accompanied by vessel augmentation, and ascites, (B) Contrast-enhanced CT images showing the omental cake, the omental tumor, and ascites.
Acknowledgements
We would like to thank Miss Sumiyo Miyakawa from Laboratory Medicine for her making bone marrow smears.
References
1. Van Nuffel AM, Sukhatme V, Pantziarka P, Meheus L, Sukhatme VP, Bouche G. Repurposing Drugs in oncology (ReDO)-clarithromycin as an anti-cancer agent. Ecancermedicalscience. 2015; 9:513. DOI: 10.3332/ecancer.2015.513.
2. Ishimatsu Y, Mukae H, Matsumoto K, Harada T, Hara A, Hara S, et al. Two cases with pulmonary mucosa-associated lymphoid tissue lymphoma successfully treated with clarithromycin. Chest. 2010; 138:730–733. DOI: 10.1378/chest.09-2358.

3. Ohe M, Hashino S. A case of follicular B-cell lymphoma treated using clarithromycin. Korean J Hematol. 2011; 46:203–206. DOI: 10.5045/kjh.2011.46.3.203.

4. Ohe M, Hashino S, Hattori A. Successful treatment of diffuse large B-cell lymphoma with clarithromycin and prednisolone. Korean J Hematol. 2012; 47:293–297. DOI: 10.5045/kjh.2012.47.4.293.

5. Coleman M, Leonard J, Lyons L, Szelenyi H, Niesvizky R. Treatment of Waldenstrom's macroglobulinemia with clarithromycin, low-dose thalidomide, and dexamethasone. Semin Oncol. 2003; 30:270–274.

6. Niesvizky R, Jayabalan DS, Christos PJ, Furst JR, Nabi T, Ely S, et al. BiRD (Biaxin (clarithromycin)/Revlimid (lenalidomide/dexamethasone) combination therapy results in high complete- and overall-response rates in treatment-naïve symptomatic multiple myeloma. Blood. 2008; 111:1101–1109.

7. Ohe M, Hashino S. Successful treatment of angioimmunoblastic T-cell lymphoma with clarithromycin. Blood Res. 2016; 51:139–142. DOI: 10.5045/br.2016.51.2.139.

8. D'Arena G, Grandone E, Di Minno MND, Musto P, Di Minno G. The anti-CD20 monoclonal antibody rituximab to treat acquired hemophilia A. Blood Transfus. 2016; 14:255–261. DOI: 10.2450/2015.0090-15.
9. Haider S, Latif T, Hochhausler A, Lucas F, Abdel Karim N. Waldenstrom's Macroglobulinemia and Peripheral Neuropathy, Organomegaly, Endocrinopathy, Monoclonal Gammopathy, and Skin Changes with a Bleeding Diathesis and Rash. Case Rep Oncol Med. 2013; 2013:890864. DOI: 10.1155/2013/890864.

10. Mamlouk MD, Vansonnenberg E, Shankar S, Silverman SG. Omental cakes: unusual aetiologies and CT appearances. Insights Imaging. 2011; 2:399–408.

11. Qi YG, Fang ZH, Huang Y. Analysis of computed tomography and pathological observations of non-Hodgkin lymphomas with peritoneal, omental and mesenteric involvement. Exp Ther Med. 2015; 9:891–894.

12. Mizunoe S, Kadota J, Tokimatsu I, Kishi K, Nagai H, Nasu M. Clarithromycin and azithromycin induce apoptosis of activated lymphocytes via down-regulation of Bcl-xL. Int Immunopharmacol. 2004; 4:1201–1207.

13. Ohara T, Morishita T, Suzuki H, Masaoka T, Ishii H, Hibi T. Antibiotics directly induce apoptosis in B cell lymphoma cells derived from BALB/c mice. Anticancer Res. 2004; 24:3723–3730.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download