Abstract
While phosphodiesterase type 5 inhibitors have been used for erectile dysfunction with acceptable safety profile, they can induce orthostatic hypotension in patients taking antihypertensive drugs with blood pressure lowering effect. This study evaluated the hemodynamic effects of 100 mg mirodenafil in hypertensive patients taking an amlodipine. Thirteen hypertensive patients who were taking 5 or 10 mg of amlodipine once daily participated in a randomized, double-blind, placebo-controlled, crossover study. A single oral dose of mirodenafil 100 mg or placebo was administered at 4.5 hour after administration of amlodipine. The maximal change in systolic and diastolic blood pressure (∆maxSBP and ∆maxDBP) and pulse rate (∆maxPR) were compared between mirodenafil and placebo periods. Twelve patients completed this study and were included analysis. The values of ∆maxPR in standing and supine position were significantly greater in the mirodenafil period (13.25±7.12 and 11.17±4.86 beats/minute) when compared to the placebo (8.50±4.72 and 6.58± 3.90 beats/minute). The ∆maxSBP and ∆maxDBP in standing position appeared to be lower in the mirodenafil period, but they were not statistically different from those in the placebo period (∆maxSBP = −7.42±5.6 vs −4.42±5.37 mmHg and ∆maxDBP = −7.17±5.72 vs −3.50±3.37 mmHg). Both ∆maxSBP and ∆maxDBP in standing and supine position were not significantly different between mirodenafil and placebo. This study demonstrated that mirodenafil exerted minimal hemodynamic effects in the patients taking amlodipine, that is unlikely associated with a clinically significant hypotensive event
References
1. Brock GB, McMahon CG, Chen KK, Costigan T, Shen W, Watkins V, et al. Efficacy and safety of tadalafil for the treatment of erectile dysfunction: results of integrated analyses. J Urol. 2002; 168:1332–1336.

2. Goldstein I, Lue TF, Padma-Nathan H, Rosen RC, Steers WD, Wicker PA. Oral sildenafil in the treatment of erectile dysfunction. Sildenafil Study Group. N Engl J Med. 1998; 338:1397–1404.
3. Hellstrom WJ, Gittelman M, Karlin G, Segerson T, Thibonnier M, Taylor T, et al. Vardenafil for treatment of men with erectile dysfunction: efficacy and safety in a randomized, double-blind, placebocontrolled trial. J Androl. 2002; 23:763–771.
4. Burchardt M, Burchardt T, Baer L, Kiss AJ, Pawar RV, Shabsigh A, et al. Hypertension is associated with severe erectile dysfunction. J Urol. 2000; 164:1188–1191.

5. Paick JS, Kim JJ, Kim SC, Moon KH, Min KS, Park K, et al. Efficacy and safety of mirodenafil in men taking antihypertensive medications. J Sex Med. 2010; 7:3143–3152. doi: 10.1111/j.1743-6109.2010.01926.x.

6. Reffelmann T, Kloner RA. Kloner, Cardiovascular effects of phosphodiesterase 5 inhibitors. Curr Pharm Des. 2006; 12:3485–3494.
7. Xin Z. [Cardiovascular safety of vardenafil]. Zhonghua Nan Ke Xue= Natl J Androl. 2004; 10:790–793.
8. Pomara G, Morelli G, Pomara S, Taddei S, Ghiadoni L, Dinelli N, et al. Cardiovascular parameter changes in patients with erectile dysfunction using pde-5 inhibitors: a study with sildenafil and vardenafil. J Androl. 2004; 25:625–629.

9. Kloner RA, Brown M, Prisant LM, Collins M. Effect of sildenafil in patients with erectile dysfunction taking antihypertensive therapy. Sildenafil Study Group. Am J Hypertens. 2001; 14:70–73.
10. Kloner RA. Pharmacology and drug interaction effects of the phosphodiesterase 5 inhibitors: focus on alpha-blocker interactions. Am J Cardiol. 2005; 96:42M–46M.
11. Chung JH, Kang DH, Oh CY, Chung JM, Lee KS, Kim TH, et al. Safety and efficacy of once daily administration of 50 mg mirodenafil in patients with erectile dysfunction: a multicenter, double-blind, placebo controlled trial. J Urol. 2013; 189:1006–1013. doi: 10.1016/j.juro.2012.08.243.

12. Park HJ, Choi HK, Ahn TY, Park JK, Chung WS, Lee SW, et al. Efficacy and safety of oral mirodenafil in the treatment of erectile dysfunction in diabetic men in Korea: a multicenter, randomized, double-blind, placebocontrolled clinical trial. J Sex Med. 2010; 7:2842–2850. doi: 10.1111/j.1743-6109.2010.01888.x.

13. Rubio-Aurioles E, Porst H, Eardley I, Goldstein I. Vardenafil-Sildenafil Comparator Study Group. Comparing vardenafil and sildenafil in the treatment of men with erectile dysfunction and risk factors for cardiovascular disease: a randomized, double-blind, pooled crossover study. J Sex Med. 2006; 3:1037–1049.
14. Jackson G, Montorsi P, Cheitlin MD. Cardiovascular safety of sildenafil citrate (Viagra): an updated perspective. Urology. 2006; 68:S47–S60.

15. Bell AS, Palmer MJ. Novel phosphodiesterase type 5 modulators: a patent survey (2008 – 2010). Expert Opin Ther Pat. 2011; 21:1631–1641. doi: 10.1517/13543776.2011.614435.

16. Lee SK, Kim Y, Kim TK, Im GJ, Lee BY, Kim DH, et al. Determination of mirodenafil and sildenafil in the plasma and corpus cavernous of SD male rats. J Pharm Biomed Anal. 2009; 49:513–518. doi: 10.1016/j.jpba.2008. 11.004.

17. Paick JS, Ahn TY, Choi HK, Chung WS, Kim JJ, Kim SC, et al. Efficacy and safety of mirodenafil, a new oral phosphodiesterase type 5 inhibitor, for treatment of erectile dysfunction. J Sex Med. 2008; 5:2672–2680. doi: 10.1111/j.1743-6109.2008.00945.x.
18. Meredith PA, Elliott HL. Clinical pharmacokinetics of amlodipine. Clin Pharmacokinet. 1992; 22:22–31.

19. Scholz H. Pharmacological aspects of calcium channel blockers. Cardiovasc Drugs Ther. 1997; 10:S869–S872.

20. Weber MA, Schiffrin EL, White WB, Mann S, Lindholm LH, Kenerson JG, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hypertens (Greenwich). 2014; 16:14–26. doi: 10.1111/jch.12237.
21. Kim BH, Yi S, Kim J, Lim KS, Kim KP, Lee B, et al. Influence of alcohol on the hemodynamic effects and pharmacokinetic properties of mirodenafil: a single-dose, randomized-sequence, open-label, crossover study in healthy male volunteers in Korea. Clin Ther. 2009; 31:1234–1243. doi: 10.1016/j.clinthera.2009.06.008.

22. Elliott HL, Green ST, Vincent J, Meredith PA. An assessment of the pharmacokinetics and pharmacodynamics of single doses of amlodipine in elderly normotensives. Pharmacol Res. 1992; 26:33–39.

23. Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, et al. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005; 45:142–161.
24. Gu N, Kim J, Lim KS, Shin KH, Kim TE, Lee B, et al. Assessment of the effect of mirodenafil on the hemodynamics of healthy male Korean volunteers administered tamsulosin: a randomized, double-blind, placebocontrolled, 2-period crossover study. Clin Ther. 2012; 34:1929–1939. doi: 10.1016/j.clinthera.2012.08.002.

25. Kloner RA, Mitchell M, Emmick JT. Cardiovascular effects of tadalafil. Am J Cardiol. 2003; 92:37M–46M.

26. EliLilly, Tadalafil prescribing information. http://pi.lilly.com/us/cialis-pi.pdf. Accessed Apr 20. 2016.
27. Kloner RA, Mitchell M, Emmick JT. Cardiovascular effects of tadalafil in patients on common antihypertensive therapies. Am J Cardiol. 2003; 92:47M–57M.

28. Perret-Guillaume C, Joly L, Benetos A. Heart rate as a risk factor for cardiovascular disease. Prog Cardiovasc Dis. 2009; 52:6–10. doi: 10.1016/j. pcad.2009.05.003.

29. van Ahlen H, Wahle K, Kupper W, Yassin A, Reblin T, Neureither M. Safety and efficacy of vardenafil, a selective phosphodiesterase 5 inhibitor, in patients with erectile dysfunction and arterial hypertension treated with multiple antihypertensives. J Sex Med. 2005; 2:856–864.

30. Pickering TG, Shepherd AM, Puddey I, Glasser DB, Orazem J, Sherman N, et al. Sildenafil citrate for erectile dysfunction in men receiving multiple antihypertensive agents: a randomized controlled trial. Am J Hypertens. 2004; 17:1135–1142.
Figure 1.
Effects of mirodenafil on hemodynamic variables while standing. Mean systolic (a) and diastolic (b) blood pressure, and pulse rate (c) following administration of amlodipine. Mirodenafil or placebo was administered at 4.5 h. All data are reported±standard deviations.
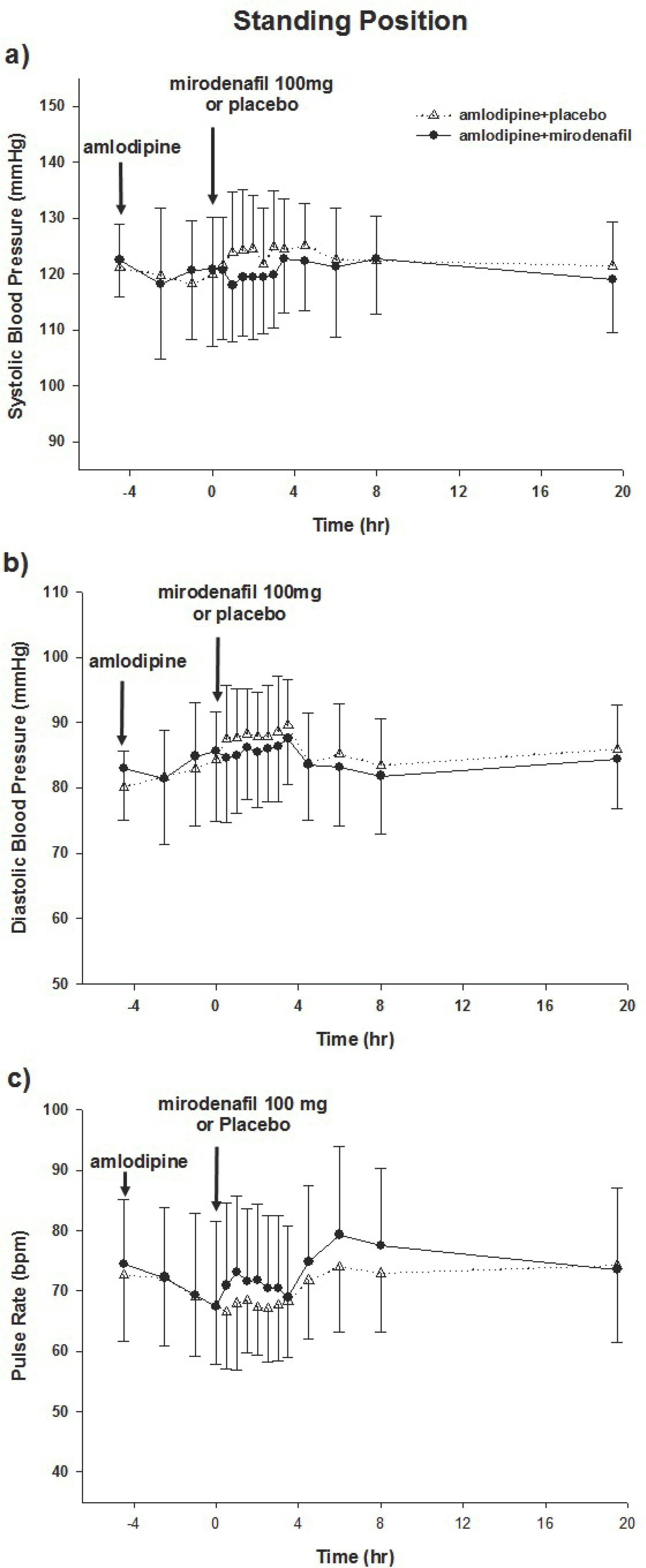
Figure 2.
Effects of mirodenafil on hemodynamic variables while supine. Mean systolic (a) and diastolic (b) blood pressure, and pulse rate (c) following administration of amlodipine. Mirodenafil or placebo was administered at 4.5 h. All data are reported±standard deviations.
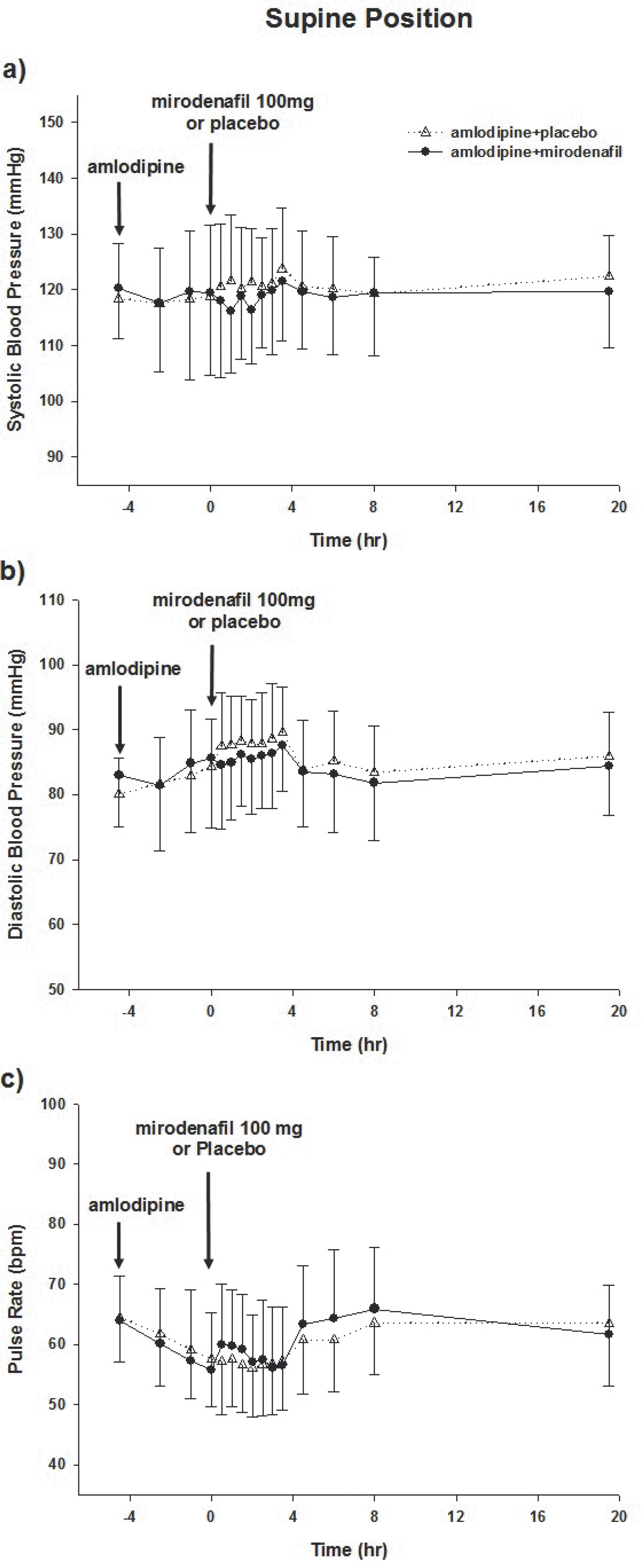
Table 1.
Baseline and effects of mirodenafil or placebo on hemodynamic variables in stable hypertensive patients taking amlodipine (n=12)
| Amlodipine + mirodenafil∗ | Amlodipine + placebo∗ | Mean difference† | p-value‡ | ||
|---|---|---|---|---|---|
| Standing | Baseline SBP | 121.1 (8.7) | 122.2 (5.3) | 0.594 | |
| Baseline DBP | 81.4 (8.0) | 81.5 (5.9) | 0.664 | ||
| Baseline PR | 74.0 (11.5) | 72.8 (10.0) | 0.400 | ||
| ∆maxSBP | –7.42 (5.60) | –4.42 (5.37) | –2.51 (−6.24∼1.22) | 0.164 | |
| ∆maxDBP | –7.17 (5.72) | –3.50 (3.37) | –3.31 (−7.17∼0.54) | 0.084 | |
| ∆maxPR | 13.25 (7.12) | 8.50 (4.72) | 5.16 (1.09∼9.22) | 0.018 | |
| Supine | Baseline SBP | 119.2 (9.9) | 119.1 (9.2) | 0.860 | |
| Baseline DBP | 74.5 (7.0) | 75.4 (7.2) | 0.824 | ||
| Baseline PR | 64.4 (7.7) | 63.7 (7.0) | 0.365 | ||
| ∆maxSBP | –6.25 (5.05) | –4.33 (7.23) | –1.33 (−6.44∼3.78) | 0.575 | |
| ∆maxDBP | –7.00 (4.67) | –5.25 (4.71) | –1.50 (−4.28∼1.28) | 0.257 | |
| ∆maxPR | 11.17 (4.86) | 6.58 (3.90) | 4.39 (1.11∼7.66) | 0.014 |
Table 2.
Calculated AUEC values of the hemodynamic variables after mirodenafil or placebo treatment in stable hypertensive patients (n=12)
| Amlodipine + Mirodenafil∗ | Amlodipine + Placebo∗ | Mean Difference† | p-value‡ | ||
|---|---|---|---|---|---|
| Standing | AUEC0∼8h, SBP | –19.65 (18.40) | –10.52 (14.45) | –7.64 (−18.81∼3.54) | 0.159 |
| AUEC0∼8h, DBP | –19.54 (18.86) | –7.44 (7.62) | –10.55 (−21.47∼0.37) | 0.057 | |
| AUEC0∼8h, PR | 56.42 (36.25) | 26.67 (19.07) | 32.28 (8.28∼54.28) | 0.013 | |
| Supine | AUEC0∼8h, SBP | –18.56 (18.42) | –13.19 (28.38) | –3.43 (−18.85∼11.99) | 0.631 |
| AUEC0∼8h, DBP | –22.25 (21.64) | –15.02 (18.31) | –6.11 (−14.77∼2.55) | 0.147 | |
| AUEC0∼8h, PR | 44.50 (21.40) | 18.73 (13.70) | 25.23 (11.75∼38.70) | 0.002 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download