Abstract
The Cmax and AUC of rosuvastatin increase when it is coadministered with telmisartan. The aim of this study was to explore which of the pharmacokinetic (PK) parameters of rosuvastatin are changed by telmisartan to cause such an interaction. We used data from drug–drug interaction (DDI) studies of 74 healthy volunteers performed in three different institutions. Rosuvastatin population PK models with or without telmisartan were developed using NONMEM (version 7.3). The plasma concentration–time profile of rosuvastatin was best described by a two-compartment, first-order elimination model with simultaneous Erlang and zero-order absorption when given rosuvastatin alone. When telmisartan was coadministered, the zero-order absorption fraction of rosuvastatin had to be omitted from the model because the absorption was dramatically accelerated. Notwithstanding the accelerated absorption, the relative bioavailability (BA) parameter estimate in the model demonstrated that the telmisartan-induced increase in BA was only about 20% and the clearance was not influenced by telmisartan at all in the final PK model. Thus, our model implies that telmisartan may influence the absorption process of rosuvastatin rather than its metabolic elimination. This may be used as a clue for further physiologically based PK (PBPK) approaches to investigate the mechanism of rosuvastatin–telmisartan DDI.
Go to : 
References
1. Rubba P, Marotta G, Gentile M. Efficacy and safety of rosuvastatin in the management of dyslipidemia. Vasc Health Risk Manag. 2009; 5:343–352.

2. Martin PD, Warwick MJ, Dane AL, Brindley C, Short T. Absolute oral bioavailability of rosuvastatin in healthy white adult male volunteers. Clin Ther. 2003; 25:2553–2563.

3. Martin PD, Mitchell PD, Schneck DW. Pharmacodynamic effects and pharmacokinetics of a new HMG-CoA reductase inhibitor, rosuvastatin, after morning or evening administration in healthy volunteers. Br J Clin Pharmacol. 2002; 54:472–477.

4. White CM. A review of the pharmacologic and pharmacokinetic aspects of rosuvastatin. J Clin Pharmacol. 2002; 42:963–970.

5. Bergman E, Forsell P, Tevell A, Persson EM, Hedeland M, Bondesson U, et al. Biliary secretion of rosuvastatin and bile acids in humans during the absorption phase. Eur J Pharm Sci. 2006; 29:205–214.

6. Kitamura S, Maeda K, Wang Y, Sugiyama Y. Involvement of multiple transporters in the hepatobiliary transport of rosuvastatin. Drug Metab Dispos. 2008; 36:2014–2023.

7. Ho RH, Tirona RG, Leake BF, Glaeser H, Lee W, Lemke CJ, et al. Drug and bile acid transporters in rosuvastatin hepatic uptake: function, expression, and pharmacogenetics. Gastroenterology. 2006; 130:1793–1806.

8. Lee E, Ryan S, Birmingham B, Zalikowski J, March R, Ambrose H, et al. Rosuvastatin pharmacokinetics and pharmacogenetics in white and Asian subjects residing in the same environment. Clin Pharmacol Ther. 2005; 78:330–341.

9. Jamei M, Bajot F, Neuhoff S, Barter Z, Yang J, Rostami-Hodjegan A, et al. A mechanistic framework for in vitro-in vivo extrapolation of liver membrane transporters: Prediction of drug-drug interaction between rosuvas-tatin and cyclosporine. Clin Pharmacokinet. 2014; 53:73–87. doi: 10.1007/s40262-013-0097-y.

10. Hua WJ, Hua WX, Fang HJ. The role of OATP1B1 and BCRP in pharmacokinetics and DDI of novel statins. Cardiovasc Ther. 2012; 30:e234–e241. doi: 10.1111/j.1755-5922.2011.00290.x.

11. Johnson ML, Pietz K, Battleman DS, Beyth RJ. Prevalence of comorbid hypertension and dyslipidemia and associated cardiovascular disease. Am J Manag Care. 2004; 10:926–932.
12. Son M, Kim Y, Lee D, Roh H, Son H, Guk J, et al. Pharmacokinetic interaction between rosuvastatin and telmisartan in healthy Korean male volunteers: a randomized, open-label, two period, crossover, multiple-dose study. Clin Ther. 2014; 36:1147–1158.
13. Tzeng TB, Schneck DW, Birmingham BK, Mitchell PD, Zhang H, Martin PD, et al. Population pharmacokinetics of rosuvastatin: implications of renal impairment, race, and dyslipidaemia. Curr Med Res Opin. 2008; 24:2575–2585. doi: 10.1185/03007990802312807.

14. Bolger MB, Lukacova V, Woltosz WS. Simulations of the nonlinear dose dependence for substrates of influx and efflux transporters in the human intestine. AAPS J. 2009; 11:353–363.

15. Jamei M, Bajot F, Neuhoff S, Barter Z, Yang J, Rostami-Hodjegan a, et al. A mechanistic framework for in vitro-in vivo extrapolation of liver membrane transporters: Prediction of drug-drug interaction between rosuvastatin and cyclosporine. Clin Pharmacokinet. 2014; 53:73–87. doi: 10.1007/s40262-013-0097-y.

16. Jones HM, Mayawala K, Poulin P. Dose selection based on physiologically based pharmacokinetic (PBPK) approaches. AAPS J. 2013; 15:377–387.

17. Gertz M, Tsamandouras N, Säll C, Houston JB, Galetin A. Reduced 233 Physiologically-Based Pharmacokinetic Model of Repaglinide: Impact of OATP1B1 and CYP2C8 Genotype and Source of In Vitro Data on the Prediction of Drug-Drug Interaction Risk. Pharm Res. 2014; 31:2367–2382.
18. Tsamandouras N, Dickinson G, Guo Y, Hall S, Rostami-Hodjegan A, Galetin A, et al. Development and Application of a Mechanistic Pharmacokinetic Model for Simvastatin and its Active Metabolite Simvastatin Acid Using an Integrated Population PBPK Approach. Pharm Res. 2015; 32:1864–1883.

19. Schneck DW, Birmingham BK, Zalikowski JA, Mitchell PD, Wang Y, Martin PD, et al. The effect of gemfibrozil on the pharmacokinetics of rosuvastatin. Clin Pharmacol Ther. 2004; 75:455–463.

20. Chauvin B, Drouot S, Barrail-Tran A, Taburet AM. Drug-drug interactions between HMG-CoA reductase inhibitors (statins) and antiviral protease inhibitors. Clin Pharmacokinet. 2013; 52:815–831.

21. Weiss J, Sauer A, Divac N, Herzog M, Schwedhelm E, Böger RH, et al. Interaction of angiotensin receptor type 1 blockers with ATP-binding cassette transporters. Biopharm Drug Dispos. 2010; 31:150–161.

22. Laporte-Simitsidis S, Girard P, Mismetti P, Chabaud S, Decousus H, Boissel JP. Inter-study variability in population pharmacokinetic metaanalysis: When and how to estimate it? J Pharm Sci. 2000; 89:155–167.
Go to : 
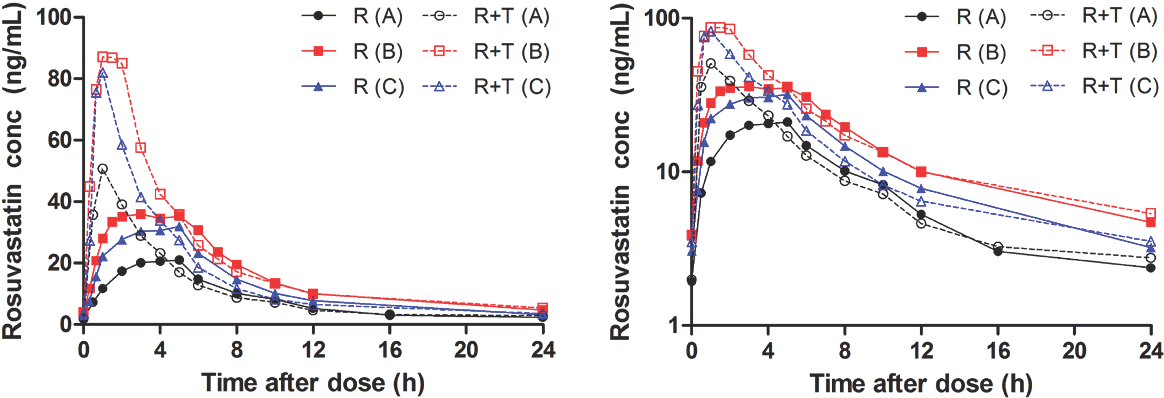 | Figure 2.Mean plasma concentration-time profiles of rosuvastatin following oral administration of rosuvastatin 20 mg alone (R) or rosuvastatin 20 mg with telmisartan 80 mg (R+T) per institution (A, B, C). Linear and semilogarithmic scales shown on left and right panels, respectively. |
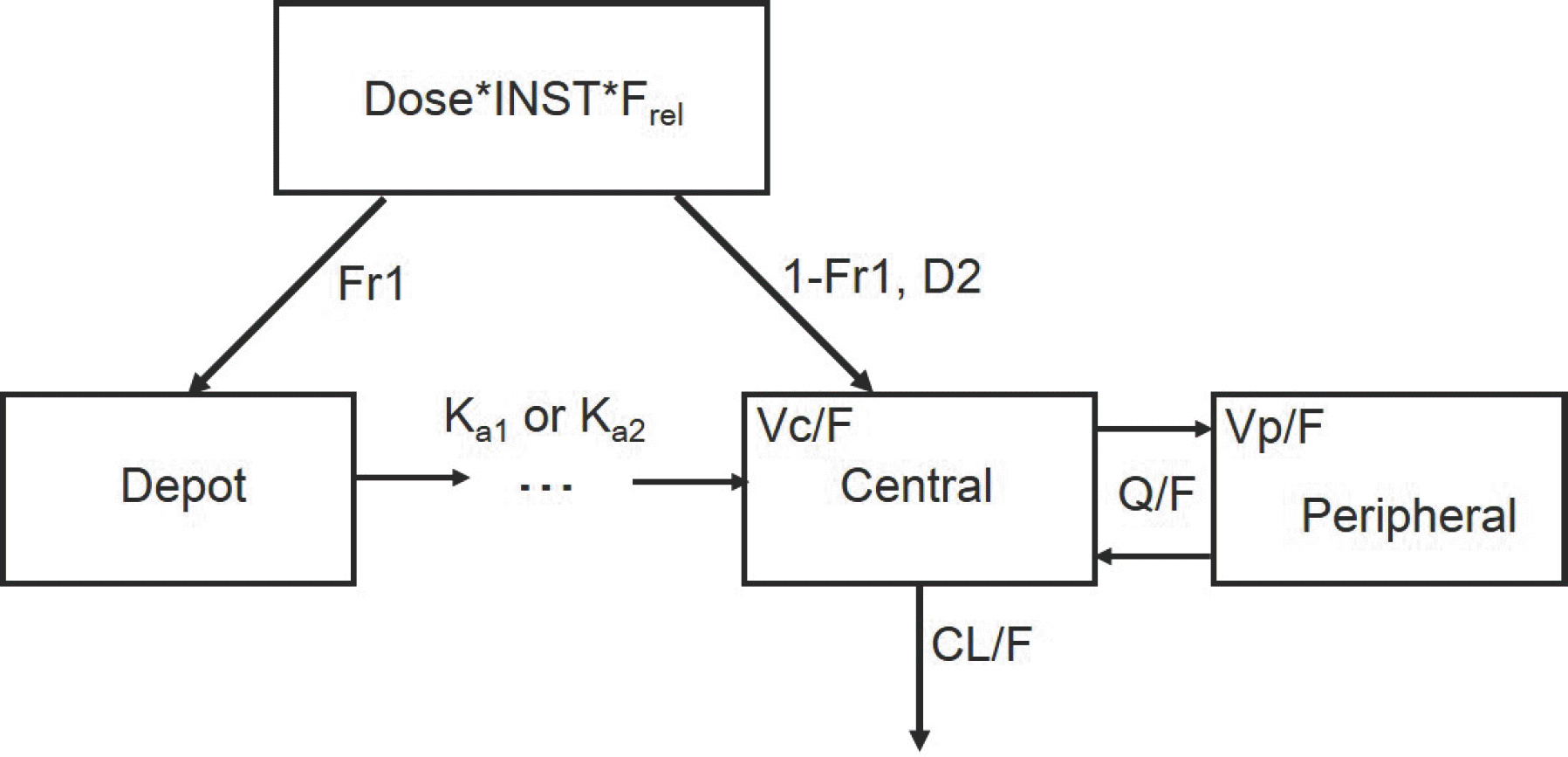 | Figure 3.Rosuvastatin PK models. INST, institutional difference (fixed as 1 for institutiona A); Frel, relative bioavailability of rosuvastatin with telmisartan (fixed as 1 for without telmisartan group); Fr1, fraction of the dose absorbed through the Erlang absorption (fixed as 1 for with telmisartan group); D2, duration of zero-order absorption (fixed as 0 for with telmisartan); Ka1, absorption rate constant without telmisartan; Ka2, absorption rate constant with telmisartan; CL/F, apparent clearance; Vc/F, apparent volume of central compartment; Q/F, apparent intercompartmental clearance; Vp/F, apparent volume of peripheral compartment. |
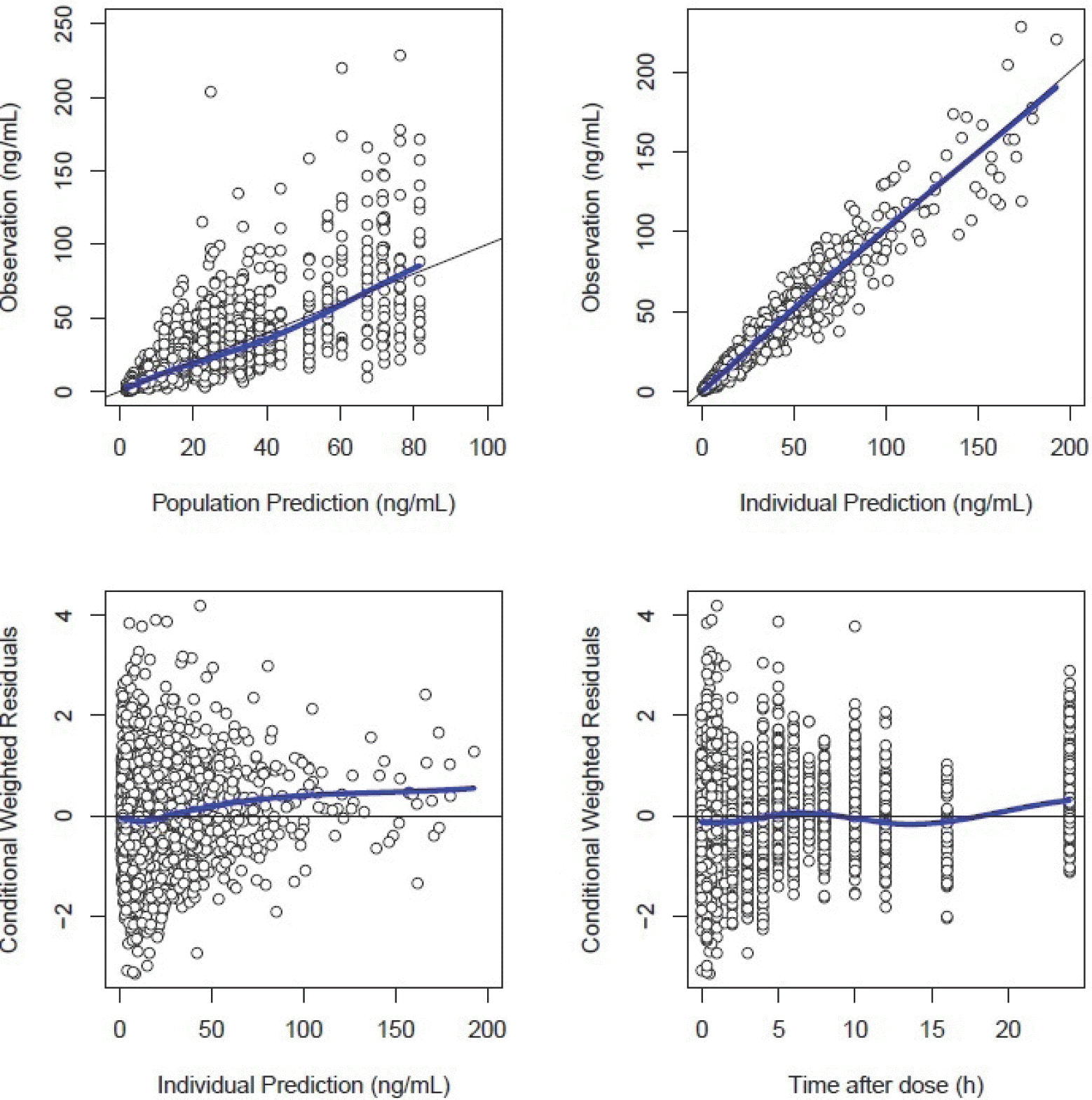 | Figure 4.Basic goodness-of-fit plots of the final model. The grey solid y = x or y = 0 lines are included for reference. The bold blue lines are the loess (local regression smoother) trend lines. |
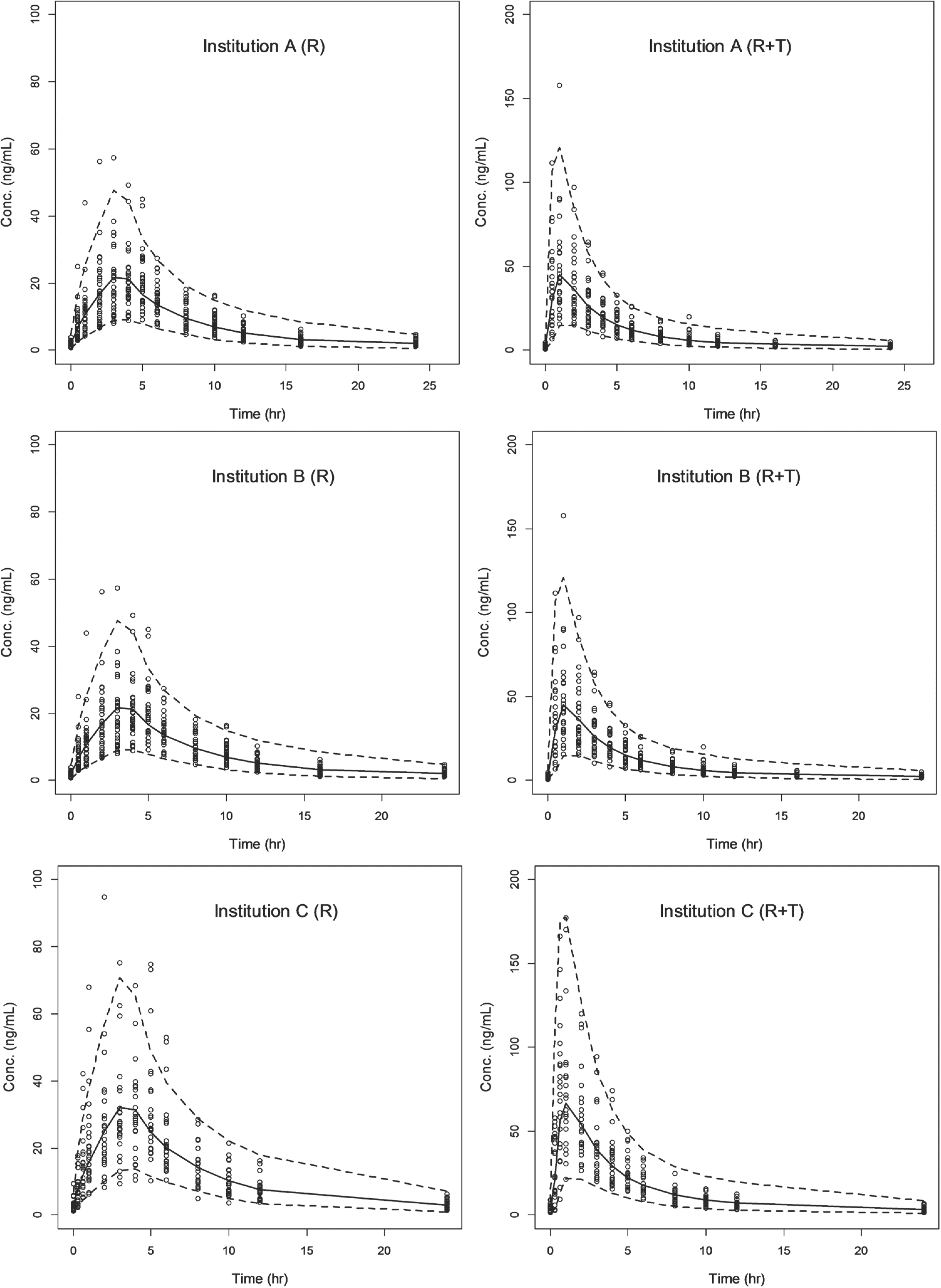 | Figure 5.Visual predictive check for the final model by each institution (Institution A, B and C) and treatment group (R; rosuvastatin alone, R+T; rosuvastatin with telmisartan). The circles show the observed rosuvastatin concentrations. The solid and dashed lines show median and 90% prediction intervals of simulation, respectively. |
Table 3.
Summary of final population PK parameter estimates
| Parameter | Description | Estimate | % RSEa | Bootstrap median (95% CI)b |
|---|---|---|---|---|
| Structural model | ||||
| CL/F (L/h) | Apparent clearance | 106 | 11.5 | 103 (88.2–111) |
| Vc/F (L) | Apparent volume of central compartment | 426 | 14.8 | 404 (339–449) |
| Q/F (L/h) | Apparent intercompartmental clearance | 48.2 | 13.0 | 47.8 (38.8–54.8) |
| Vp/F (L) | Apparent volume of peripheral compartment | 829 | 16.6 | 778 (634–969) |
| Without Telmisartan | ||||
| Ka1 (h−1) | Absorption rate constant of Erlang absorption without telmisartan | 0.910 | 11.8 | 0.912 (0.815–0.974) |
| Fr1 | Fraction absorbed by Erlang absorption | 0.197 | 20.7 | 0.200 (0.160–0.238) |
| D2 (h) | Duration of dosing for zero-order absorption | 3.56 | 4.8 | 3.56 (3.41–3.75) |
| With Telmisartan | ||||
| Ka2 (h−1) | Absorption rate constant of Erlang absorption with telmisartan | 9.77 | 8.0 | 9.82 (8.61–11.2) |
| Frel | Relative bioavailability of rosuvastatin with telmisartan when that without telmisartan assumed to be 1 | 1.30 | 4.3 | 1.29 (1.23–1.37) |
| Inter-study difference (INST) | ||||
| Inst_A | Fixed study difference of Inst_A | 1.0 (Fixed) | – | – |
| Inst_B | Fixed study difference of Inst_B compared to Inst_A | 1.58 | 9.1 | 1.54 (1.19–1.96) |
| Inst_C | Fixed study difference of Inst_C compared to Inst_A | 1.48 | 9.6 | 1.44 (1.15–1.87) |
| Inter-individual variability | ||||
| ωCL/F (%) | Interindividual variability of CL/F | 43.3 | 13.1 | 40.9 (34.6–46.7) |
| ωVc/F (%) | Interindividual variability of Vc/F | 64.8 | 11.7 | 58.7 (49.3–66.3) |
| ωVp/F (%) | Interindividual variability of Vp/F | 67.1 | 16.0 | 61.1 (49.0–73.3) |
| ωQ/F (%) | Interindividual variability of Q/F | 60.6 | 11.9 | 54.0 (41.7–68.3) |
| ωka1 (%) | Interindividual variability of Ka1 | 19.7 | 38.6 | 19.8 (0.40–40.6) |
| ωka2 (%) | Interindividual variability of Ka2 | 49.0 | 14.5 | 45.8 (37.7–53.9) |
| ωFrel (%) | Interindividual variability of Frel | 22.0 | 13.1 | 21.7 (16.7–25.0) |
| ρCL/F∼Vc/F | Correlation coefficient between CL/F and Vc/F | 0.920 | 12.9 | 0.919 (0.874–0.952) |
| ρCL/F∼Vp/F | Correlation coefficient between CL/F and Vp/F | 0.941 | 14.2 | 0.945 (0.880–0.999) |
| ρVc/F∼Vp/F | Correlation coefficient between Vc/F and Vp/F | 0.971 | 12.7 | 0.973 (0.900–1.000) |
| Residual error | ||||
| σadd (ng/mL) | Additive error | 0.292 | 19.6 | 0.289 (0.103–0.406) |
| σprop | Proportional error | 0.202 | 1.8 | 0.201 (0.190–0.217) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download