Abstract
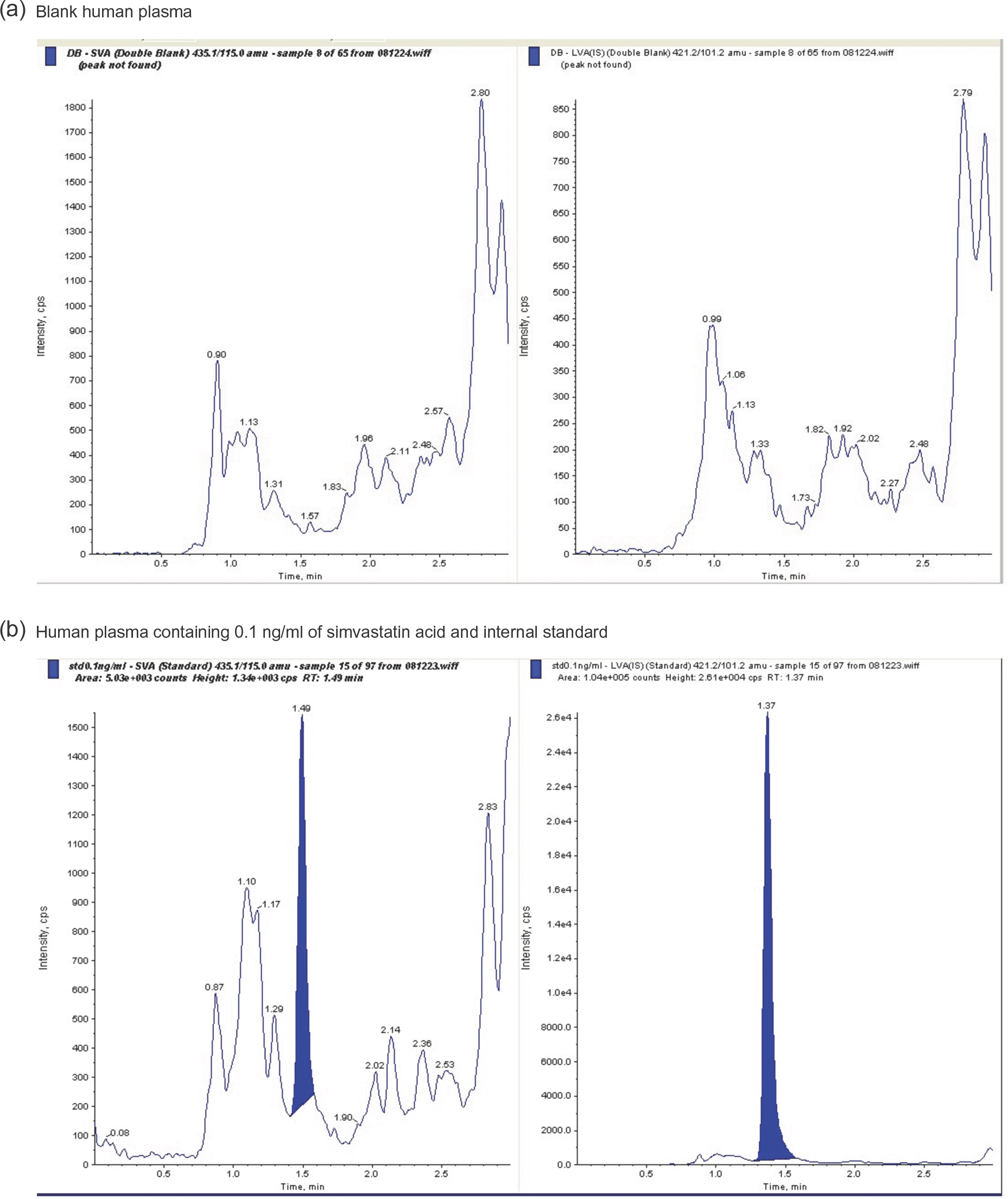
Simvastatin is a lipid-lowering drug that is metabolized to its active metabolite simvastatin acid (SA). We developed and validated a sensitive liquid chromatography–tandem mass spectrometry (LC/MS/MS) method to quantitate SA in human plasma using a liquid–liquid extraction method with methanol. The protonated analytes generated in negative ion mode were monitored by multiple reaction monitoring. Using 500-mL plasma aliquots, SA was quantified in the range of 0.1–100 ng/mL. Calibration was performed by internal standardization with lovastatin acid, and regression curves were generated using a weighting factor of 1/x². The linearity, precision, and accuracy of this assay for each compound were validated using quality control samples consisting of mixtures of SA (0.1, 0.5, 5, and 50 ng/mL) and plasma. The intra-batch accuracy was 95.3–107.8%, precision was –2.2% to –3.7%, and linearity (r²) was over 0.998 in the standard calibration range. The chromatographic running time was 3.0 min. This method sensitively and reliably measured SA concentrations in human plasma and was successfully used in clinical pharmacokinetic studies of simvastatin in healthy Korean adult male volunteers.
Go to : 
References
1. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994; 344:1383–1389.
2. HMG-CoA reductase inhibitors for hypercholesterolemia. N Engl J Med. 1988; 319:1222–1223.
3. Taylor F, Huffman MD, Macedo AF, Moore TH, Burke M, Davey Smith G, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013; 1:CD004816.

4. Minder CM, Blaha MJ, Horne A, Michos ED, Kaul S, Blumenthal RS. Evidence-based use of statins for primary prevention of cardiovascular disease. Am J Med. 2012; 125:440–446. doi: 10.1016/j.amjmed.2011.11.013.

5. Prueksaritanont T, Gorham LM, Ma B, Liu L, Yu X, Zhao JJ, et al. In vitro metabolism of simvastatin in humans [SBT] identification of metabolizing enzymes and effect of the drug on hepatic P450s. Drug Metab Dispos. 1997; 25:1191–1199.
6. Prueksaritanont T, Ma B, Yu N. The human hepatic metabolism of simvastatin hydroxy acid is mediated primarily by CYP3A, and not CYP2D6. Br J Clin Pharmacol. 2003; 56:120–124.

7. Matusewicz L, Meissner J, Toporkiewicz M, Sikorski AF. The effect of statins on cancer cells–review. Tumour Biol. 2015 Jul; 36:4889–4904. doi: 10. 1007/s13277-015-3551-7.

8. Merck Sharpe & Dohme. Manufacturer Information, Zocor: simvastatin, West Point, PA. 1991.
9. Prueksaritanont T, Subramanian R, Fang X, Ma B, Qiu Y, Lin JH, et al. Glucuronidation of statins in animals and humans: a novel mechanism of statin lactonization. Drug Metab Dispos. 2002; 30:505–512.

10. Prueksaritanont T, Qiu Y, Mu L, Michel K, Brunner J, Richards KM, et al. Interconversion pharmacokinetics of simvastatin and its hydroxy acid in dogs: effects of gemfibrozil. Pharm Res. 2005; 22:1101–1109.

Go to : 
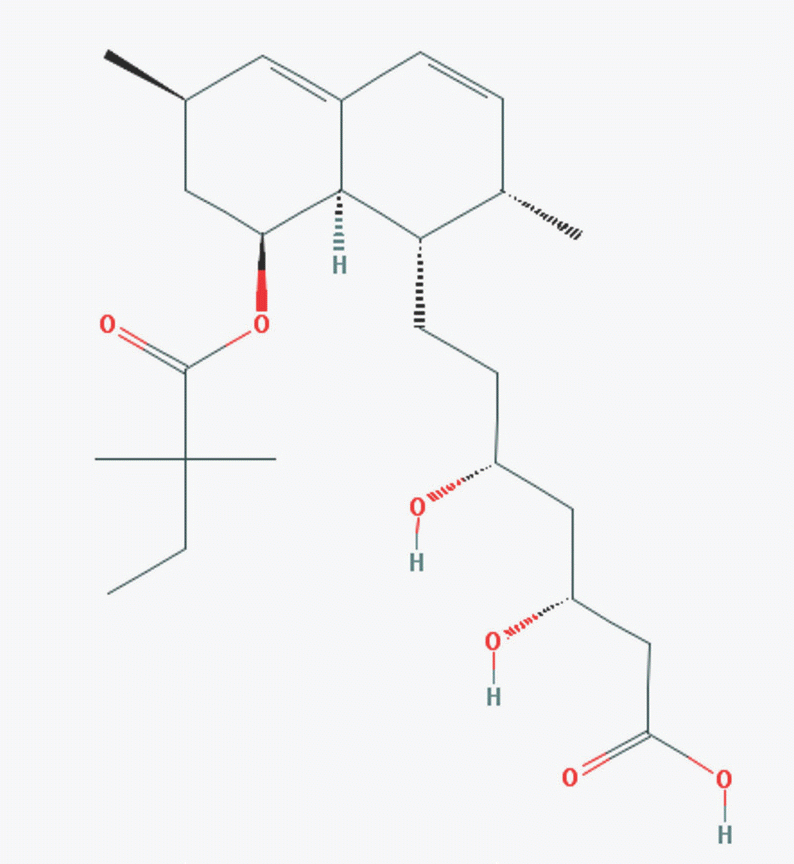 | Figure 1.Chemical structure of simvastatin acid (Source: http://pub-chem.ncbi.nlm.nih.gov/compound/64718#section=Top). |
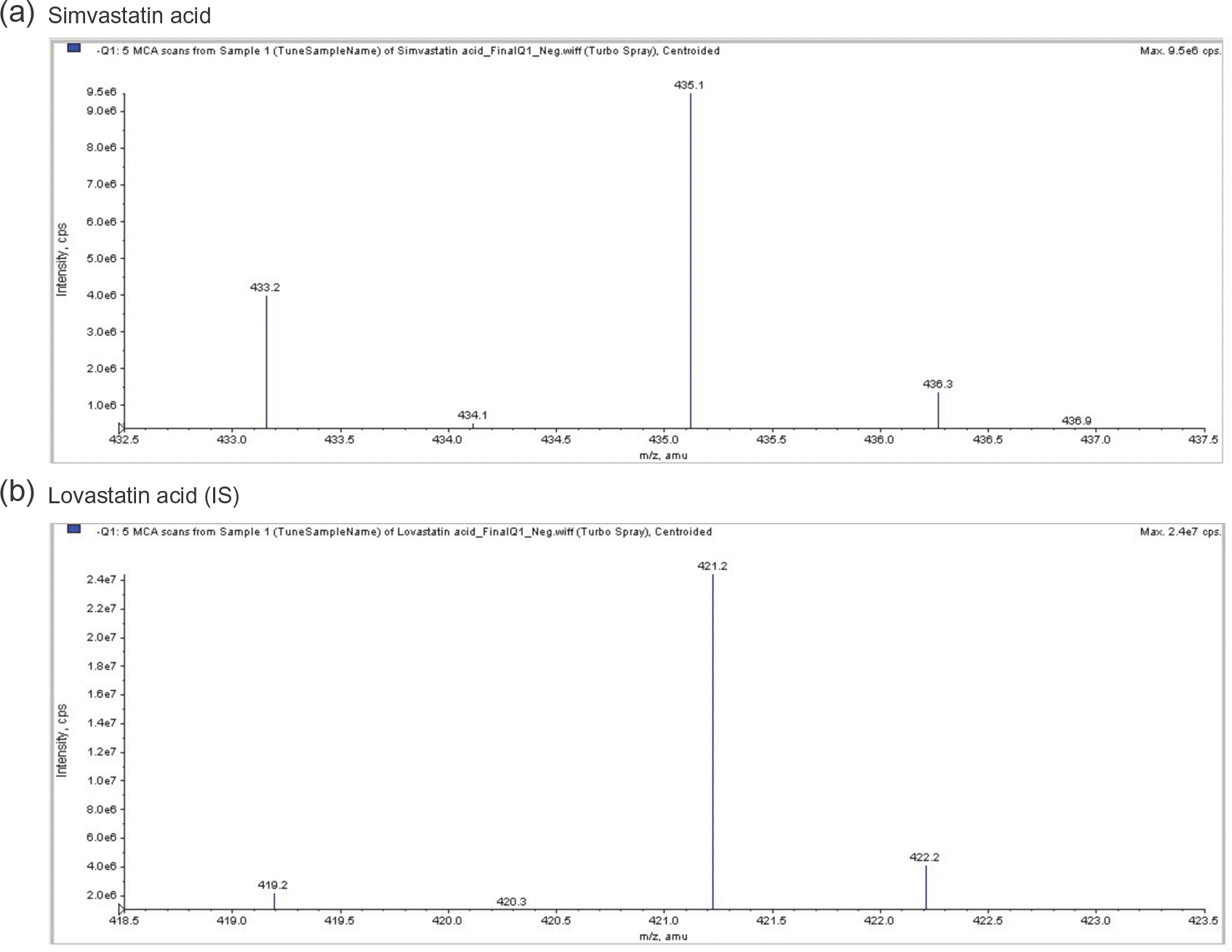 | Figure 2.Full-scan product ion spectra of [M−H]+ for simvastatin acid and the lovastatin acid internal standard. |
Table 1.
Tandem mass spectrometry parameters
| Analyte | MRM transition (m/z) | Time (msec) | DP∗ (volts) | EP∗ (volts) | CE∗ (volts) | CXP∗ (volts) |
|---|---|---|---|---|---|---|
| Simvastatin acid | 435.12 > 115.0 | 150 | –90 | –8 | –36 | –7 |
| Lovastatin acid | 435.12 > 115.0 | 150 | –70 | –8 | –36 | –5 |
Table 2.
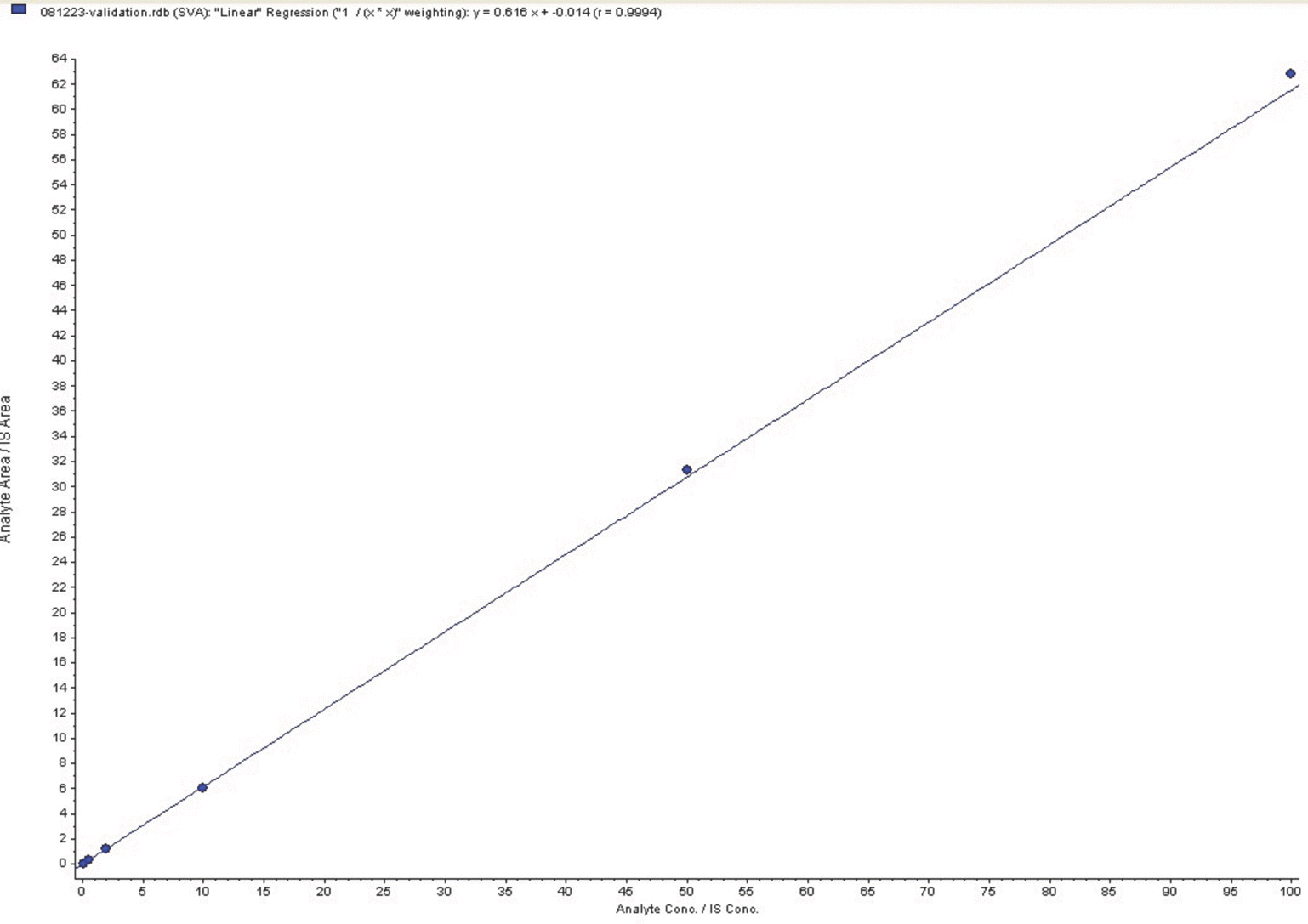
Inter-batch precision and accuracy for calibration of standard data
| Analyte | Nominal concentration (ng/mL) | Mean calculated concentration‡ (ng/mL) | Accuracy∗ (%) | Precision† (%) |
|---|---|---|---|---|
| Simvastatin | 0.10 | 0.10 | 100.8 | 0.9 |
| acid | 0.50 | 0.47 | 94.9 | 3.5 |
| 2.00 | 2.06 | 103.1 | 2.2 | |
| 10.00 | 10.27 | 102.7 | 2.4 | |
| 50.00 | 49.56 | 99.1 | 2.2 | |
| 100.00 | 99.32 | 99.3 | 3.3 |
Table 3.
Intra- and inter-batch precision and accuracy of quality control samples in human plasma
| Intra-day (n=5) | Inter-day (n=5) | ||||||
|---|---|---|---|---|---|---|---|
| Analyte | Nominal Conc. (ng/mL) | Mean calculated Conc.‡ (ng/mL) | Accuracy∗ (%) | Precision† (%) | Mean calculated Conc.‡ (ng/mL) | Accuracy∗ (%) | Precision† (%) |
| Simvastatin | 0.10 | 0.10 | 104.6 | 3.7 | 0.10 | 103.2 | 5.7 |
| acid | 0.50 | 0.48 | 95.3 | 2.9 | 0.47 | 93.5 | 3.5 |
| 5.00 | 5.38 | 107.6 | 2.2 | 4.95 | 99.1 | 2.23 | |
| 50.00 | 53.86 | 107.8 | 3.7 | 50.22 | 100.4 | 4.7 | |
Table 4.
Stability of simvastatin acid in human plasma samples




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download