Abstract
Alpha-lipoic acid, a physiological form of thioctic acid, is a strong antioxidant that relieves diabetic neuropathic symptoms. R(+)-α-lipoic acid shows superior antioxidative effects to its racemate. We compared the pharmacokinetics (PKs) and tolerability of R(+)- and S(-)-α-lipoic acid after a single oral dose of R(+)-α-lipoic acid, Dexid®, and its racemate, thioctic acid in healthy male subjects. We used an open-label, randomized, single-dose, three-treatment, parallel study design to compare the PK exposure of the active form, R(+)-α-lipoic acid. Thirty subjects completed the study with no clinically relevant safety issues. The peak concentrations (Cmax, mean±SD) of R(+)-α-lipoic acid after doses of R(+)-α-lipoic acid 200 mg, 300 mg and thioctic acid 600 mg were 4186.8±1956.7, 6985.6±3775.8 and 6498.4±3575.6 μg/L, respectively, and the areas under the plasma concentration-time curve from 0 to the last measurable concentration (AUClast) were 1893.6±759.4, 3575.2±1149.2 and 3790.0±1623.0 μg·h-1·L-1, respectively. The geometric mean ratio and 90% confidence intervals of R(+)-α-lipoic acid 200 mg to thioctic acid 600 mg for the Cmax and AUClast were 0.71 (0.43–1.15) and 0.51 (0.37–0.70), respectively. The corresponding R(+)-α-lipoic acid 300 mg to thioctic acid 600 mg values were 1.11 (0.68-1.80) and 0.97 (0.71-1.34), respectively. In conclusion, R(+)-α-lipoic acid 300 mg showed PK characteristics similar to those of thioctic acid 600 mg and both formulations were well tolerated.
REFERENCES
1.Kandhwal K., Dey S., Nazarudheen S., Arora R., Reyar S., Thudi NR, et al. Pharmacokinetics of a Fixed-Dose Combination of Atorvastatin and Metformin Extended Release versus Concurrent Administration of Individual Formulations. Clin Drug Investig. 2011. 31:853–863. DOI: doi: 10.1007/bf032569 23.

2.Ziegler D. Treatment of diabetic neuropathy and neuropathic pain: how far have we come? Diabetes Care. 2008. 31:S255–S261. DOI: doi: 10.2337/dc08-s263.
3.Wong MC., Chung JW., Wong TK. Effects of treatments for symptoms of painful diabetic neuropathy: systematic review. BMJ. 2007. 335:87. DOI: doi: 10. 1136/bmj.39213.565972.AE.

4.Reljanovic M., Reichel G., Rett K., Lobisch M., Schuette K., Möller W, et al. Treatment of diabetic polyneuropathy with the antioxidant thioctic acid (α-lipoic acid): A two year multicenter randomized double-blind placebo-controlled trial (ALADIN II). Free Radic Res. 1999. 31:171–179. DOI: doi: 10.1080/10715769900300721.

5.Tomassoni D., Amenta F., Di Cesare Mannelli L., Ghelardini C., Nwankwo IE., Pacini A, et al. Neuroprotective activity of thioctic acid in central nervous system lesions consequent to peripheral nerve injury. Biomed Res Int. 2013. 2013:985093. DOI: doi: 10.1155/2013/985093.

6.Borcea V., Nourooz-Zadeh J., Wolff SP., Klevesath M., Hofmann M., Urich H, et al. alpha-Lipoic acid decreases oxidative stress even in diabetic patients with poor glycemic control and albuminuria. Free Radic Biol Med. 1999. 26:1495–1500.
7.Carlson DA., Smith AR., Fischer SJ., Young KL., Packer L. The plasma pharmacokinetics of R-(+)-lipoic acid administered as sodium R-(+)-lipoate to healthy human subjects. Altern Med Rev. 2007. 12:343–351.
8.Mignini F., Streccioni V., Tomassoni D., Traini E., Amenta F. Comparative crossover, randomized, open-label bioequivalence study on the bioequivalence of two formulations of thioctic acid in healthy volunteers. Clin Exp Hypertens. 2007. 29:575–586. DOI: doi: 10.1080/10641960701744111.

9.Teichert J., Hermann R., Ruus P., Preiss R. Plasma kinetics, metabolism, and urinary excretion of alpha-lipoic acid following oral administration in healthy volunteers. J Clin Pharmacol. 2003. 43:1257–1267. DOI: doi: 10.1177/0091270003258654.

10.Hong YS., Jacobia SJ., Packer L., Patel MS. The inhibitory effects of lipoic compounds on mammalian pyruvate dehydrogenase complex and its catalytic components. Free Radic Biol Med. 1999. 26:685–694.

11.Packer L., Tritschler HJ., Wessel K. Neuroprotection by the metabolic antioxidant α-lipoic acid. Free Radic Biol Med. 1997. 22:359–378. DOI: doi: 10.1016/s0891-5849(96)00269-9.

12.Hermann R., Niebch G., Borbe HO., FiegerBuschges H., Ruus P., Nowak H, et al. Enantioselective pharmacokinetics and bioavailability of different racemic alpha-lipoic acid formulations in healthy volunteers. Eur J Pharm Sci. 1996. 4:167–174. DOI: doi: Doi 10.1016/0928-0987(95)00045-3.
13.Mijnhout GS., Kollen BJ., Alkhalaf A., Kleefstra N., Bilo HJ. Alpha lipoic Acid for symptomatic peripheral neuropathy in patients with diabetes: a meta-analysis of randomized controlled trials. Int J Endocrinol. 2012. 2012:456279. DOI: doi: 10.1155/2012/456279.

14.Kroemer HK., Fromm MF., Bühl K., Terefe H., Blaschke G., Eichelbaum M. An enantiomer-enantiomer interaction of (S)- and (R)-propafenone modifies the effect of racemic drug therapy. Circulation. 1994. 89:2396–2400.

15.Nguyen LA., He H., Pham-Huy C. Chiral drugs: an overview. Int J Biomed Sci. 2006. 2:85–100.
Figure 2.
Mean plasma concentration-time profiles of R(+)-α-lipoic acid after a single oral administration of R(+)-α-lipoic acid 200 mg or 300 mg or thioctic acid 600 mg. Inset shows profile by log-linear scale. Bars represent SD. (∗ N=2 for R(+)-α-lipoic acid 200 mg, N=7 for R(+)-α-lipoic acid 300 mg and thioctic acid 600 mg)
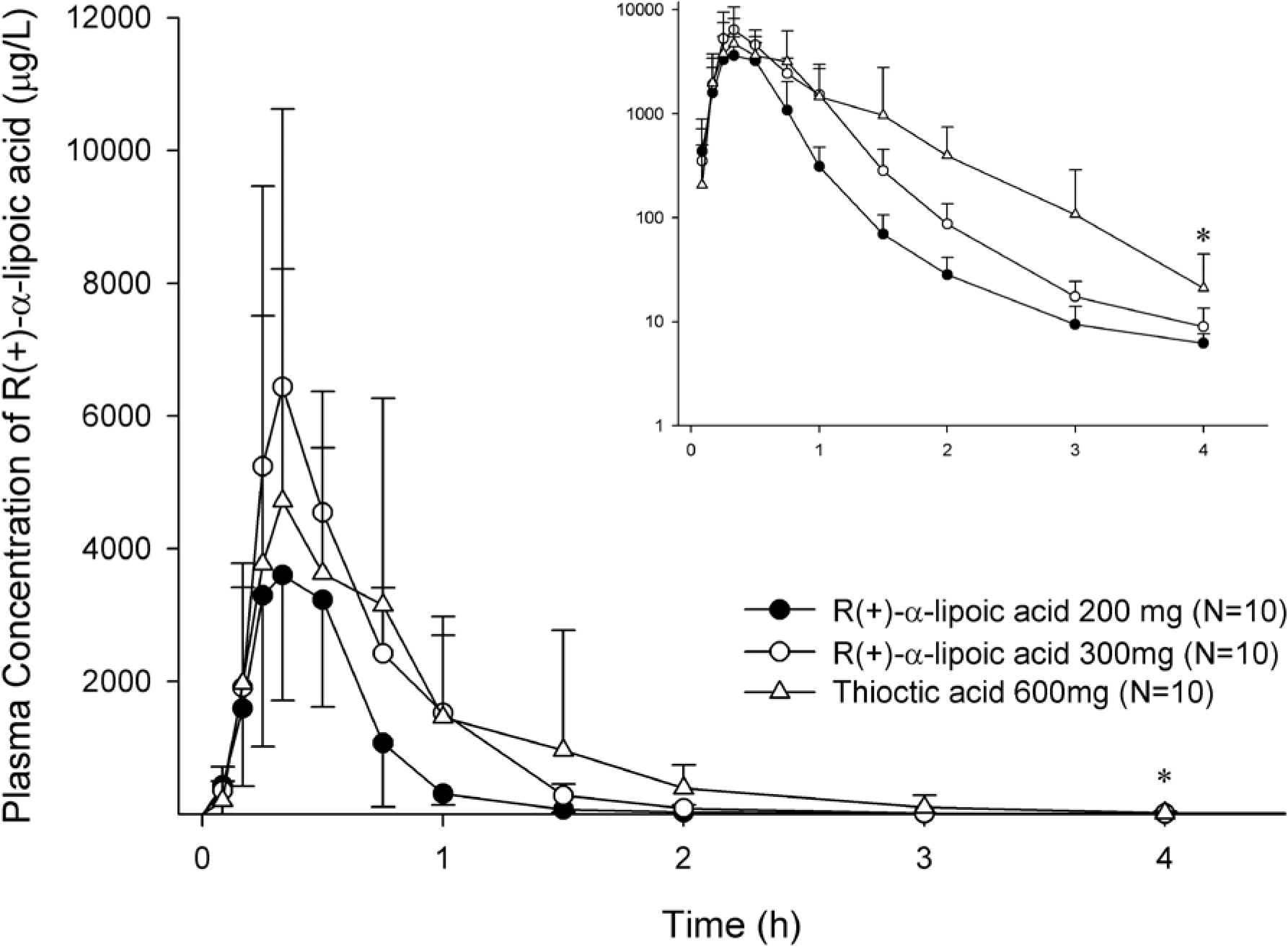
Figure 3.
Comparison of peak concentration (Cmax, upper) and area under the plasma concentration-time curve from 0 to the last measurable concentration (AUClast, lower) of R(+)-α-lipoic acid after a single oral administration of R(+)-α-lipoic acid 200 mg or 300 mg or thioctic acid 600 mg. Boxes indicate interquartile range and whisker bars indicate 10th and 90th percentiles. Horizontal bars locate in the middle of the boxes represent median and the long-dashed line shows mean.
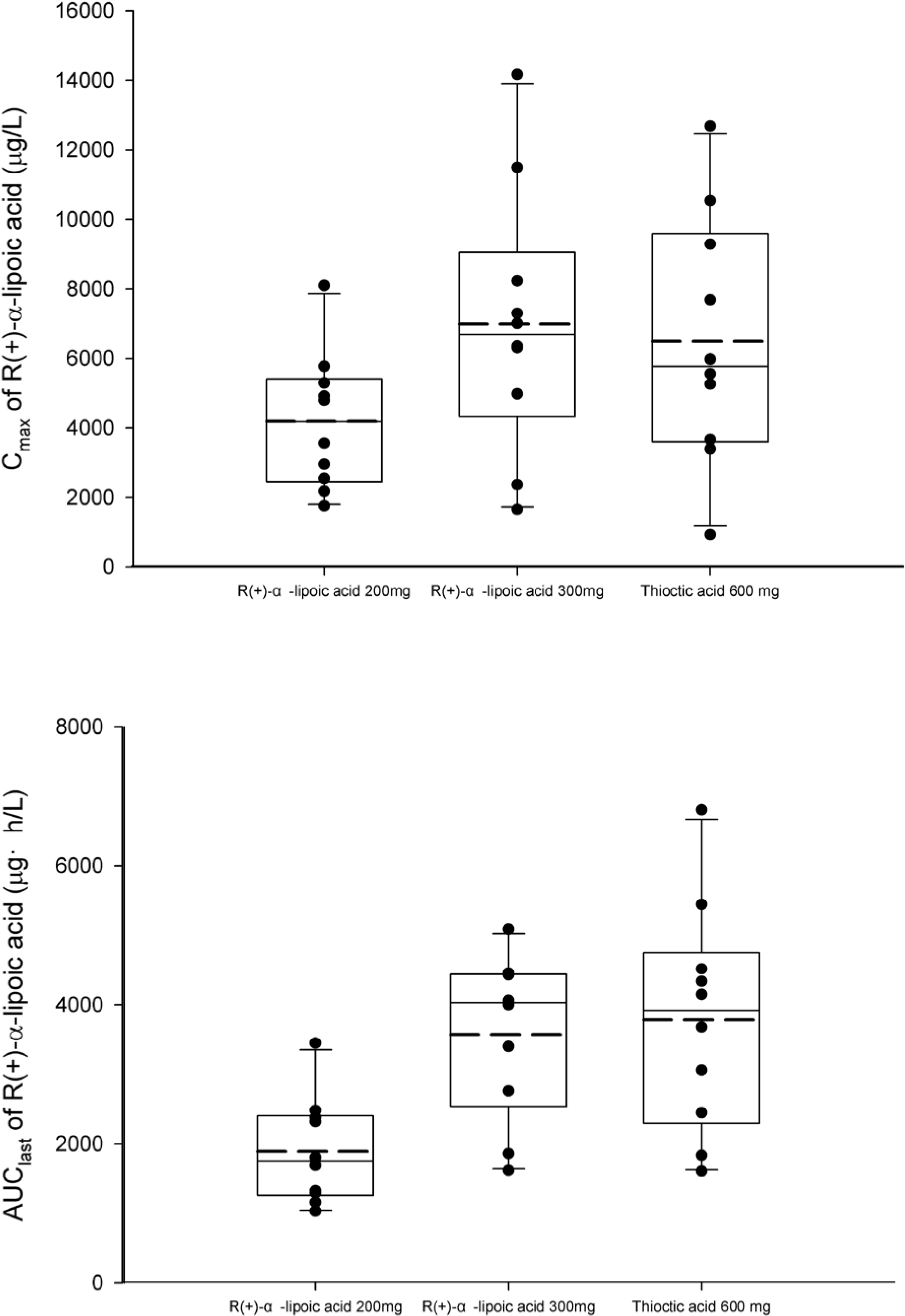
Table 1.
Demographic characteristics of the study subjects
| Treatment | |||||
|---|---|---|---|---|---|
| R(+)-α-lipoic acid 200 mg (Dexid® 320 mg) (N=10) | R(+)-α-lipoic acid 300 mg (Dexid® 480 mg) (N=10) | Thioctic acid 600 mg (N=10) | Total (N=30) | p-value1) | |
| Age (years) | 25.3±4.9 | 25.2±5.2 | 23.2±1.8 | 24.6±4.2 | 0.78 |
| Height (cm) | 174.6±4.5 | 172.0±6.5 | 174.5±5.6 | 173.7±5.5 | 0.58 |
| Weight (kg) | 68.0±6.3 | 64.5±9.3 | 68.8±5.1 | 67.1±7.1 | 0.17 |
Table 2.
Summary of Pharmacokinetic Parameters of R(+)-α-lipoic acid and S(-)-α-lipoic acid after a single oral administration of R(+)-α-lipoic acid 200 mg, 300 mg or thioctic acid 600 mg
| Parameters∗ | R(+)-α-lipoic acid 200 mg (Dexid® 320 mg) (N=10) | R(+)-α-lipoic acid 300 mg (Dexid® 480 mg) (N=10) | Thioctic acid 600 mg (N=10) | ||||
|---|---|---|---|---|---|---|---|
| R(+)-α-lipoic acid | S(-)-α-lipoic acid | R(+)-α-lipoic acid | S(-)-α-lipoic acid | R(+)-α-lipoic acid | S(-)-α-lipoic acid | ||
| tmax (h) | Median Range | 0.33 (0.25-0.75) | - | 0.33 (0.25-1.00) | - | 0.33 (0.25-0.75) | 0.33 (0.25-0.75) |
| Cmax (µg/L) | Mean±SD | 4186.8±1956.7 | - | 6985.6±3775.8 | - | 6498.4±3575.6 | 3552.6±1942.4 |
| Range | (1761.0-8099.0) | - | (1655.0-14170.0) | - | (930.1-12680.0) | (532.8-2908.2) | |
| AUClast (µg·h/L) | Mean±SD | 1893.6±759.4 | - | 3575.2±1149.2 | - | 3790.0±1623.0 | 1969.4±1031.5 |
| Range | (1031.2-3448.0) | - | (1622.7-5085.3) | - | (1610.4-6807.3) | (812.3-3770.5) | |
| AUC0-∞ (µg·h/L) | Mean±SD | 1900.7±758.9 | - | 3585.5±1146.0 | - | 3805.0±1623.7 | 2073.6±951.8 |
| Range | (1038.6-3454.0) | - | (1627.8-5091.9) | - | (1621.3-6833.4) | (851.5-3777.9) | |
| t1/2 (h) | Mean±SD | 0.7±0.2 | 0.7±0.3 | 0.6±0.2 | 0.9±1.4 | ||
| Range | (0.5-1.1) | - | (0.4-1.2) | - | (0.3-0.9) | (0.3-4.8) | |
| CL/F (L/h) | Mean±SD | 120.4±44.6 | - | 95.5±42.6 | - | 95.3±46.9 | 175.5±83.3 |
| Range | (57.9-192.6) | - | (58.9-184.3) | - | (43.9-185.0) | (79.4-352.3) | |
∗ Cmax: the peak plasma concentration, tmax: time to maximum plasma concentration, AUClast: the area under the plasma concentration versus time curve from 0 to the last measurable concentration, AUC0-∞: the area under the plasma concentration versus time curve from 0 extrapolated to infinity, t1/2: terminal half-life, CL/F: apparent clearance.
Table 3.
Comparison of pharmacokinetic parameters after a single oral administration of R(+)-α-lipoic acid 200 mg, 300 mg or thioctic acid 600 mg




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download