Abstract
Cigarette smoking may be associated with the augmentation of pro-inflammatory cytokines including Tumor Necrosis Factor-alpha (TNF-α), which may affect the outcomes of pharmacological agents such as TNF-α inhibitors. The purpose of this study was to investigate the impact of smoking on the effectiveness of TNF-α inhibitors in patients with rheumatoid arthritis (RA) or Crohn's disease (CD). We used systematic literature review methods. A total of 1,147 articles were selected after exclusion of duplicates through a database search. Among them, 28 articles were finally selected through a review of titles and abstracts and a subsequent review of full articles. The effectiveness of TNF-α inhibitors in patients with RA or CD among the selected articles was summarized by their smoking status. Meta-analysis was performed with random effect model. When current smokers were compared with non-smokers for response after adjustments through meta-analysis among patients with RA, current smokers had 59% less response than non-smokers with statistical significance (Pooled adjusted OR=0.41, 95% CI=0.17–0.95). In patients with CD, current smokers tended to have lower clinical response than non-smokers, but statistical significance was not shown. In subgroup analyses for luminar CD or fistulizing CD, current smokers tended to have a lower response in luminar CD (Pooled OR=0.62, 95% CI=0.34–1.14), but smoking status was not associated with drug response in fistulizing CD. This study raises awareness of the adverse effects of smoking in terms of clinical response in patients treated with TNF-α inhibitors.
Go to : 
References
2. Arnson Y, Shoenfeld Y, Amital H. Effects of tobacco smoke on immunity, inflammation and autoimmunity. J Autoimmun. 2010; 34:J258–265. doi: 10.1016/j.jaut.2009.12.003.

3. Källberg H, Ding B, Padyukov L, Bengtsson C, Ronnelid J, Klareskog L, et al. Smoking is a major preventable risk factor for rheumatoid arthritis: estimations of risks after various exposures to cigarette smoke. Ann Rheum Dis. 2011; 70:508–511. doi: 10.1136/ard.2009.120899.

4. Nos P, Domenech E. Management of Crohn's disease in smokers: is an alternative approach necessary? World J Gastroenterol. 2011; 17:3567–3574. doi: 10.3748/wjg.v17.i31.3567.

5. Finckh A, Ciurea A, Brulhart L, Möller B, Walker UA, Courvoisier D, et al. Which subgroup of patients with rheumatoid arthritis benefits from switching to rituximab versus alternative anti-tumour necrosis factor (TNF) agents after previous failure of an anti-TNF agent? Ann Rheum Dis. 2010; 69:387–393. doi: 10.1136/ard.2008.105064.

6. Narula N, Fedorak RN. Does smoking reduce infliximab's effectiveness against Crohn's disease? Can J Gastroenterol. 2009; 23:121–125.

7. Abhishek A, Butt S, Gadsby K, Zhang W, Deighton CM. Anti-TNF-alpha agents are less effective for the treatment of rheumatoid arthritis in current smokers. J Clin Rheumatol. 2010; 16:15–18. doi: 10.1097/RHU.0b013e3181ca4a2a.
8. Canhão H, Rodrigues AM, Mourão AF, Martins F, Santos MJ, Canas-Silva J, et al. Comparative effectiveness and predictors of response to tumour necrosis factor inhibitor therapies in rheumatoid arthritis. Rheumatology (Oxford). 2012; 51:2020–2026. doi: 10.1093/rheumatology/kes184.
9. Hyrich KL, Watson KD, Silman AJ, Symmons DP. Predictors of response to anti-TNF-alpha therapy among patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register. Rheumatology (Oxford). 2006; 45:1558–1565.
10. Mattey DL, Brownfield A, Dawes PT. Relationship between pack-year history of smoking and response to tumor necrosis factor antagonists in patients with rheumatoid arthritis. J Rheumatol. 2009; 36:1180–1187. doi: 10.3899/jrheum.081096.

11. Saevarsdottir S, Wedrén S, Seddighzadeh M, Bengtsson C, Wesley A, Lindblad S, et al. Patients with early rheumatoid arthritis who smoke are less likely to respond to treatment with methotrexate and tumor necrosis factor inhibitors: observations from the Epidemiological Investigation of Rheumatoid Arthritis and the Swedish Rheumatology Register cohorts. Arthritis Rheum. 2011; 63:26–36. doi: 10.1002/art.27758.
12. Söderlin MK, Petersson IF, Geborek P. The effect of smoking on response and drug survival in rheumatoid arthritis patients treated with their first anti-TNF drug. Scand JRheumatol. 2012; 41:1–9. doi: 10.3109/03009742.2011.599073.

13. Hlavaty T, Pierik M, Henckaerts L, Ferrante M, Joossens S, van Schuer-beek N, et al. Polymorphisms in apoptosis genes predict response to infliximab therapy in luminal and fistulizing Crohn's disease. Aliment Pharmacol Ther. 2005; 22:613–626.

14. Katz L, Gisbert JP, Manoogian B, Lin K, Steenholdt C, Mantzaris GJ, et al. Doubling the infliximab dose versus halving the infusion intervals in Crohn's disease patients with loss of response. Inflamm Bowel Dis. 2012; 18:2026–2033. doi: 10.1002/ibd.22902.

15. Aguas M, Bastida G, Cerrillo E, Beltrán B, Iborra M, Sánchez-Montes C, et al. Adalimumab in prevention of postoperative recurrence of Crohn's disease in high-risk patients. World J Gastroenterol. 2012; 18:4391–4398. doi: 10.3748/wjg.v18.i32.4391.

16. Arnott ID, McNeill G, Satsangi J. An analysis of factors influencing short-term and sustained response to infliximab treatment for Crohn's disease. Aliment Pharmacol Ther. 2003; 17:1451–1457.

17. Chaparro M, Panes J, García V, Mañosa M, Esteve M, Merino O, et al. Long-term durability of infliximab treatment in Crohn's disease and efficacy of dose "escalation" in patients losing response. J Clin Gastroenterol. 2011; 45:113–118. doi: 10.1097/MCG.0b013e3181ebaef9.

18. Fefferman DS, Lodhavia PJ, Alsahli M, Falchuk KR, Peppercorn MA, Shah SA, et al. Smoking and immunomodulators do not influence the response or duration of response to infliximab in Crohn's disease. Inflamm Bowel Dis. 2004; 10:346–351.

19. Laharie D, Salzmann M, Boubekeur H, Richy F, Amouretti M, Quinton A, et al. Predictors of response to infliximab in luminal Crohn's disease. Gastroenterol Clin Biol. 2005; 29:145–149.

20. Luna-Chadid M, Pérez Calle JL, Mendoza JL, Vera MI, Bermejo AF, Sánchez F, et al. Predictors of response to infliximab in patients with fistulizing Crohn's disease. Rev Esp Enferm Dig. 2004; 96:379–381. ,382–384.

21. Kevans D, Keegan D, Mulcahy HE, O'Donoghue DP. Infliximab therapy in Crohn's disease: A pragmatic approach? Aliment Pharmacol Ther. 2006; 24:351–359.

22. Kong JY, Bundell C, Pawlik J, Hollingsworth P, Forbes G. Low Trough serum infliximab and antibodies to infliximab in smokers. Inflamm Bowel Dis. 2013; 19:E35–E36. doi: 10.1002/ibd.22928.

23. Lin KK, Velayos F, Fisher E, Terdiman JP. Durability of infliximab dose intensification in crohn's disease. Dig Dis Sci. 2012; 57:1013–1019. doi: 10. 1007/s10620-011-1969-3.

24. Molnár T, Farkas K, Nyári T, Szepes Z, Nagy F, Wittmann T. Frequency and predictors of loss of response to infliximab or adalimumab in Crohn's disease after one-year treatment period – a single center experience. J Gastrointestin Liver Dis. 2012; 21:265–269.
25. Nichita C, Stelle M, Vavricka S, El-Wafa Ali A, Ballabeni P, De Saussure P, et al. Clinical experience with adalimumab in a multicenter swiss cohort of patients with crohn's disease. Digestion. 2010; 81:78–85. doi: 10.1159/000253855.

26. Orlando A, Colombo E, Kohn A, Biancone L, Rizzello F, Viscido A, et al. Infliximab in the treatment of Crohn's disease: Predictors of response in an Italian multicentric open study. Dig Liver Dis. 2005; 37:577–583.

27. Papamichael K, Archavlis E, Lariou C, Mantzaris GJ. Adalimumab for the prevention and/or treatment of postoperative recurrence of Crohn's disease: A prospective, two-year, single center, pilot study. J Crohns Colitis. 2012; 6:924–931. doi: 10.1016/j.crohns.2012.02.012.

28. Parsi MA, Achkar JP, Richardson S, Katz J, Hammel JP, Lashner BA, et al. Predictors of response to infliximab in patients with Crohn's disease. Gastroenterology. 2002; 123:707–713.

29. Parsi MA, Lashner BA, Achkar JP, Connor JT, Brzezinski A. Type of Fistula Determines Response to Infliximab in Patients with Fistulous Crohn's Disease. Am J Gastroenterol. 2004; 99:445–449.

30. Rudolph SJ, Weinberg DI, McCabe RP. Long-term durability of Crohn's disease treatment with infliximab. Dig Dis Sci. 2008; 53:1033–1041.

31. Sakuraba A, Sato T, Matsukawa H, Okamoto S, Takaishi H, Ogata H, et al. The use of infliximab in the prevention of postsurgical recurrence in poly-surgery Crohn's disease patients: A pilot open-labeled prospective study. Int J Colorectal Dis. 2012; 27:947–952. doi: 10.1007/s00384-011-1398-y.

32. Triantafillidis JK, Mantzaris G, Karagiannis J, Papavasilliou E, Pa-patheodoridis G, Fouskas J, et al. Similar response to adalimumab in patients with active Crohn's disease either naive to biologic agents or with prior loss of response or intolerance to infliximab. Rev Med Chir Soc Med Nat Iasi. 2010; 114:85–90.
33. Vermeire S, Louis E, Carbonez A, Van Assche G, Noman M, Belaiche J, et al. Demographic and clinical parameters influencing the short-term outcome of antitumor necrosis factor (infliximab) treatment in Crohn's disease. Am J Gastroenterol. 2002; 97:2357–2363.

34. Zorzi F, Zuzzi S, Onali S, Calabrese E, Condino G, Petruzziello C, et al. Efficacy and safety of infliximab and adalimumab in Crohn's disease: a single centre study. Aliment Pharmacol Ther. 2012; 35:1397–1407.

35. Mitchell P, Mok T, Barraclough H, Strizek A, Lew R, van Kooten M. Smoking history as a predictive factor of treatment response in advanced nonsmall-cell lung cancer: a systematic review. Clin Lung Cancer. 2012; 13:239–251. doi: 10.1016/j.cllc.2011.08.003.

36. Hamilton JD, Thomson EA, Porter D, Hunter JA, Madhok R, Capell HA. Lifestyle influences on outcome in rheumatoid arthritis. Scott Med J. 2000; 45:137–139.

37. Harrison BJ, Silman AJ, Wiles NJ, Scott DG, Symmons DP. The association of cigarette smoking with disease outcome in patients with early in-flammatory polyarthritis. Arthritis Rheum. 2001; 44:323–330.

38. Harel-Meir M, Sherer Y, Shoenfeld Y. Tobacco smoking and autoimmune rheumatic diseases. Nat Clin Pract Rheumatol. 2007; 3:707–715.

39. Glossop JR, Dawes PT, Mattey DL. Association between cigarette smoking and release of tumour necrosis factor alpha and its soluble receptors by peripheral blood mononuclear cells in patients with rheumatoid arthritis. Rheumatology (Oxford). 2006; 45:1223–1229.
Go to : 
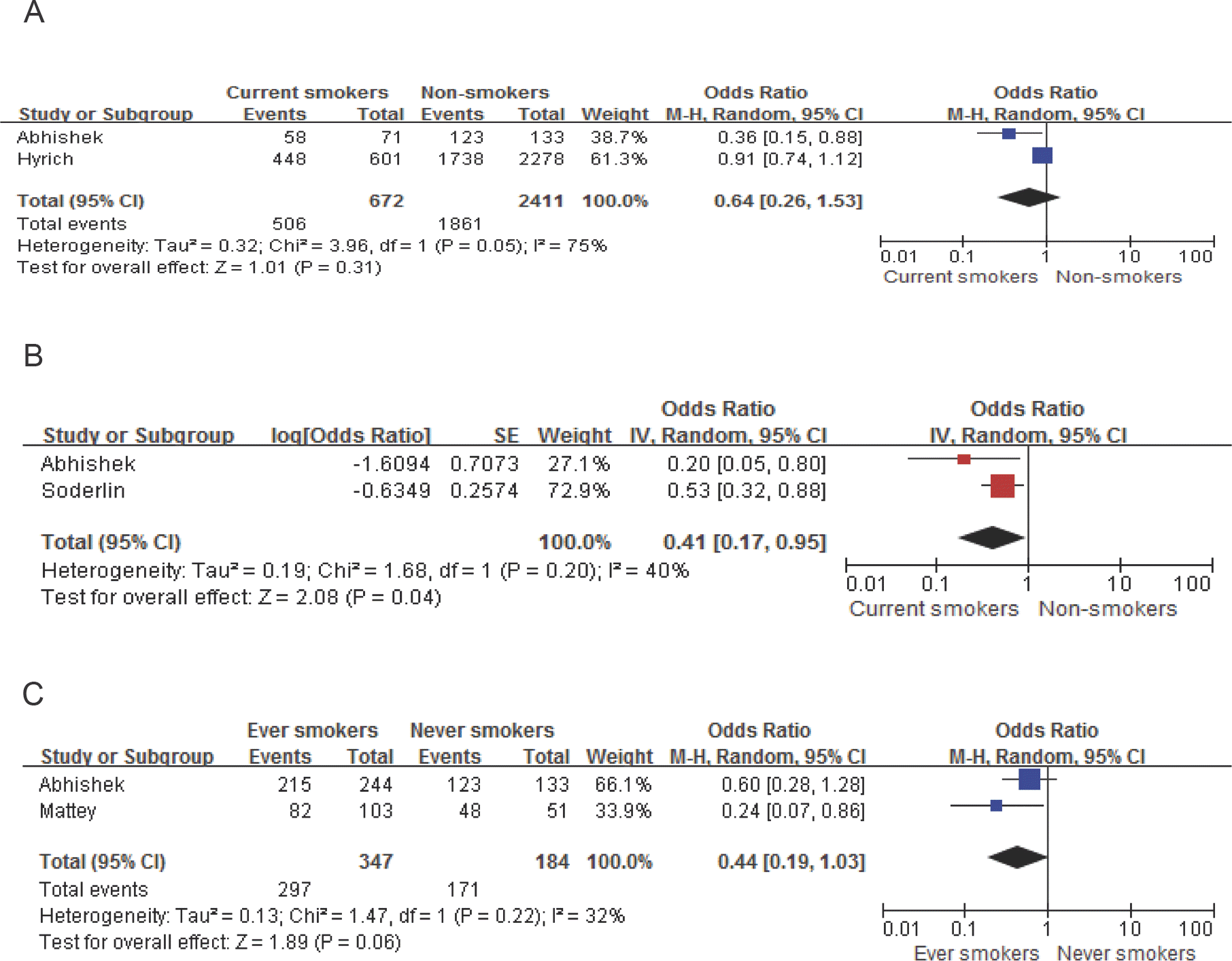 | Figure 2.Meta-analysis of clinical response to TNF-α inhibitors in patients with rheumatoid arthritis. A. current smokers vs. non-smokers, B. current smokers vs. non-smokers after adjustment, C. ever-smokers vs. never-smokers |
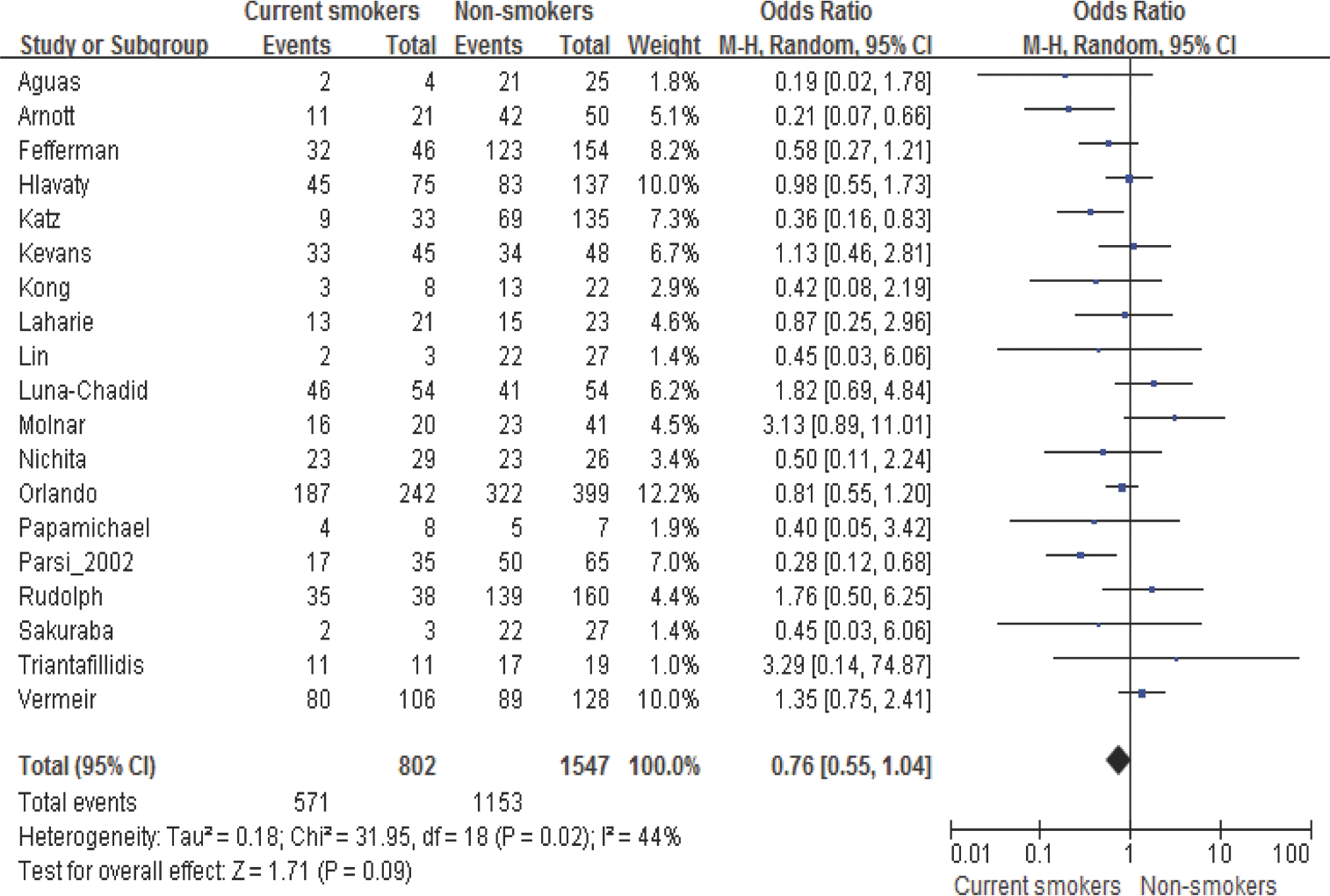 | Figure 3.Meta-analysis of clinical response to TNF-α inhibitors between current smokers and non-smokers in patients with Crohn's disease |
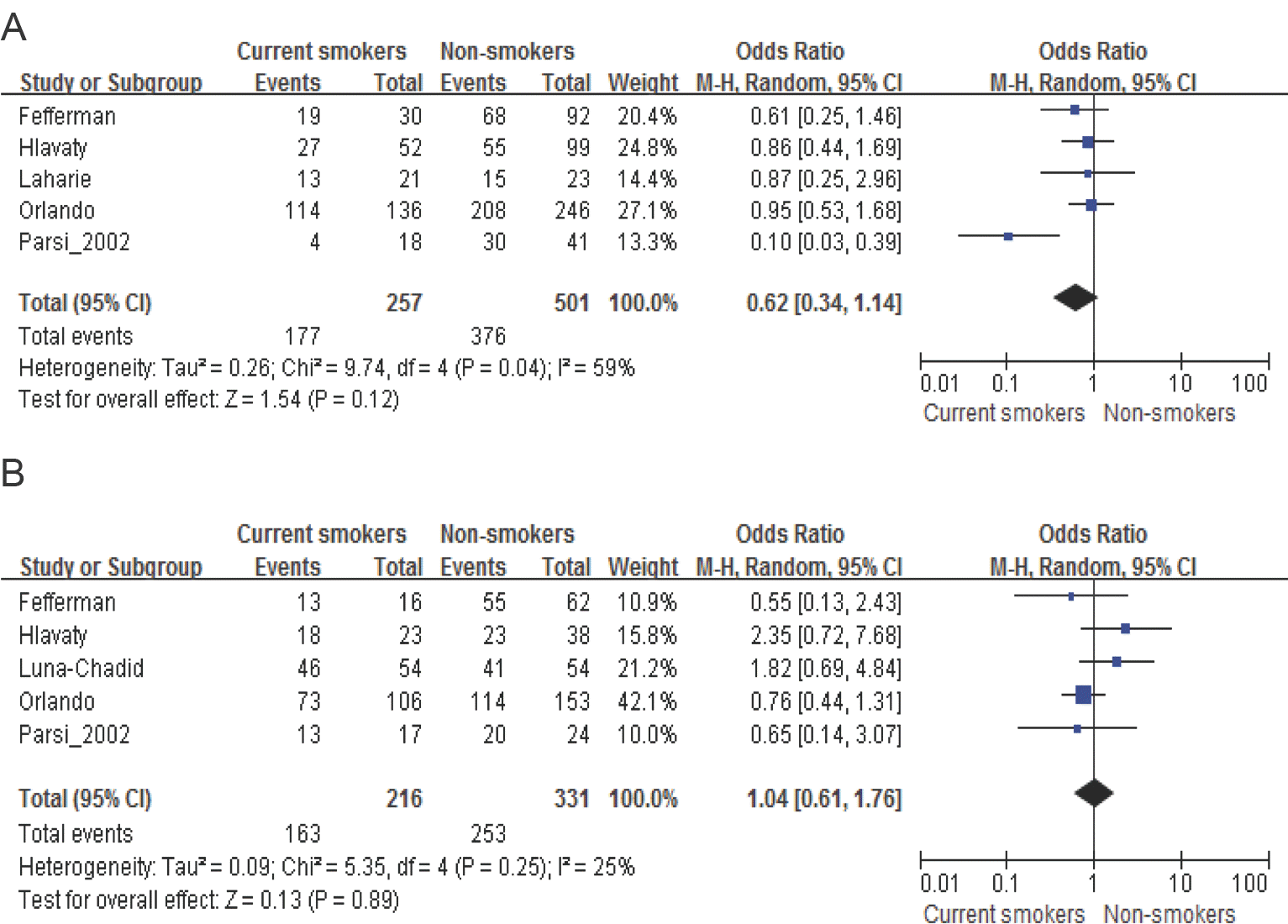 | Figure 4.Meta-analysis of clinical response to TNF-α inhibitors in patients with luminar or fistulizing Crohn's disease. A. current smokers vs. non-smokers in patients with luminar Crohn's disease, B. current smokers vs. nonsmokers in patients with fistulizing Crohn's disease |
Table 1.
Summary of clinical response to TNF-alpha inhibitors in patients with rheumatoid arthritis
Table 2.
Summary of clinical response to TNF-alpha inhibitors in patients with Crohn's disease




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download