Abstract
SKL10406, triple monoamine reuptake inhibitor, is a novel antidepressant candidate. A PET study was performed to investigate the occupancies of serotonin and dopamine transporters (SERT and DAT) in human brain, and the relationship between SKL10406 concentration and SERT occupancy was assessed using pharmacokinetic-pharmacodynamic (PK-PD) modeling methods. Fifteen healthy volunteers were given SKL10406 100 mg/day for 6 days or 150 mg/day for 6 days after 100 mg/day for 4 days. Each subject underwent full PK sampling for SKL10406 and PET scans at pre-dose, 4 h and 16 h after dosing at a steady state to investigate the occupancies of SERT and DAT using11C-DASB and11C-PE2I, respectively. Naïve pooled method (NPM) and nonlinear mixed-effect methods (ME) including a direct ME (DME) and an effect compartmental ME (EME) were used (NONMEM Ver. 7.2). Six and five subjects completed the studies for SERT and DAT, respectively. The final estimates of Emax (53.4%) and EC50 (11.8 ng/mL) from DME were relatively lower than those from NPM (Emax, 74.1%; EC50, 36.8 ng/mL) and EME (Emax, 68.6%; EC50, 40.2 ng/mL). DAT occupancy results were not modeled because of lower occupancies. The results showed that the dosage regimens may be applied in patient studies. However, difference between estimation methods alerts that ME may not be a recommendable analysis tool for sparsely sampled PET scan data.
Go to : 
References
2. Peeters M, Giuliano F. Central neurophysiology and dopaminergic control of ejaculation. Neurosci Biobehav Rev. 2008; 32:438–453.

3. Fava M, Rankin M. Sexual functioning and SSRIs. J Clin Psychiatry. 2002; 63(Suppl 5):13–16.
4. Gitlin MJ, Suri R, Altshuler L, Zuckerbrow-Miller J, Fairbanks L. Bupropion-sustained release as a treatment for SSRI-induced sexual side effects. J Sex Marital Ther. 2002; 28:131–138.

5. Han S, Ryu EJ, Lee KH, Park CE, Shin YJ. A novel serotonin-preferred triple reuptake inhibitor, SKL10406, has competitive and tolerable anti-depression profile. Eur Neuropsychopharmacol. 2012; 22:S272–S273.
6. Farde L. The advantage of using positron emission tomography in drug research. Trends Neurosci. 1996; 19:211–214.

7. Waarde Av. Measuring receptor occupancy with PET. Curr Pharm Des. 2000; 6:1593–1610.
8. Kim E, Howes OD, Kim BH, Jeong JM, Lee JS, Jang IJ, et al. Predicting brain occupancy from plasma levels using PET: superiority of combining pharmacokinetics with pharmacodynamics while modeling the relationship. J Cereb Blood Flow Metab. 2012; 32:759–768. doi: 10.1038/jcbfm. 2011.180.

9. Berges A, Cunningham VJ, Gunn RN, Zamuner S. Non linear mixed effects analysis in PET PK-receptor occupancy studies. NeuroImage. 2013; 76:155–166. doi: 10.1016/j.neuroimage.2013.03.006.

10. Abanades S, van der Aart J, Barletta JA, Marzano C, Searle GE, Salinas CA, et al. Prediction of repeat-dose occupancy from single-dose data: characterisation of the relationship between plasma pharmacokinetics and brain target occupancy. J Cereb Blood Flow Metab. 2011; 31:944–952. doi: 10.1038/jcbfm.2010.175.

11. Houle S, Ginovart N, Hussey D, Meyer JH, Wilson AA. Imaging the serotonin transporter with positron emission tomography: initial human studies with [11C]DAPP and [11C]DASB. Eur J Nucl Med. 2000; 27:1719–1722.
12. Meyer JH, Wilson AA, Ginovart N, Goulding V, Hussey D, Hood K, et al. Occupancy of serotonin transporters by paroxetine and citalopram during treatment of depression: a [11C]DASB PET imaging study. Am J Psychiatry. 2001; 158:1843–1849.
13. Meyer JH, Wilson AA, Sagrati S, Hussey D, Carella A, Potter WZ, et al. Serotonin transporter occupancy of five selective serotonin reuptake inhibitors at different doses: an [11C]DASB positron emission tomography study. Am J Psychiatry. 2004; 161:826–835.
14. Wilson AA, Ginovart N, Schmidt M, Meyer JH, Threlkeld PG, Houle S. Novel radiotracers for imaging the serotonin transporter by positron emission tomography: synthesis, radiosynthesis, and in vitro and ex vivo evaluation of 11C-labeled 2-(phenylthio)araalkylamines. J Med Chem. 2000; 43:3103–3110.
15. Dolle F, Bottlaender M, Demphel S, Emond P, Fuseau C, Coulon C, et al. Highly efficient synthesis of [11C]PE2I, a selective radioligand for the quantification of the dopamine transporter using PET. J Labelled Comp Radiopharm. 2000; 43:997–1004.
16. Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996; 16:834–840.

17. Pinborg LH, Videbaek C, Svarer C, Yndgaard S, Paulson OB, Knudsen GM. Quantification of [123I]PE2I binding to dopamine transporters with SPET. Eur J Nucl Med Mol Imaging. 2002; 29:623–631.
18. Suhara T, Takano A, Sudo Y, Ichimiya T, Inoune M, Yasuno F, et al. High levels of serotonin transporter occupancy with low-dose clomipramine in comparative occupancy study with fluvoxamine using positron emission tomography. Arch Gen Psychiatry. 2003; 60:386–391.

19. Nogami T, Takano H, Arakawa R, Ichimiya T, Fujiwara H, Kimura Y, et al. Occupancy of serotonin and norepinephrine transporter by milnacipran in patients with major depressive disorder: a positron emission tomography study with [(11)C]DASB and (S,S)-[(18)F]FMeNER-D(2). Int J Neuropsychopharmacol. 2013; 16:937–943. doi: 10.1017/S1461145712001009.

20. Areberg J, Luntang-Jensen M, Sogaard B, Nilausen DO. Occupancy of the serotonin transporter after administration of Lu AA21004 and its relation to plasma concentration in healthy subjects. Basic Clin Pharmacol Toxicol. 2012; 110:401–404. doi: 10.1111/j.1742-7843.2011.00810.x.

Go to : 
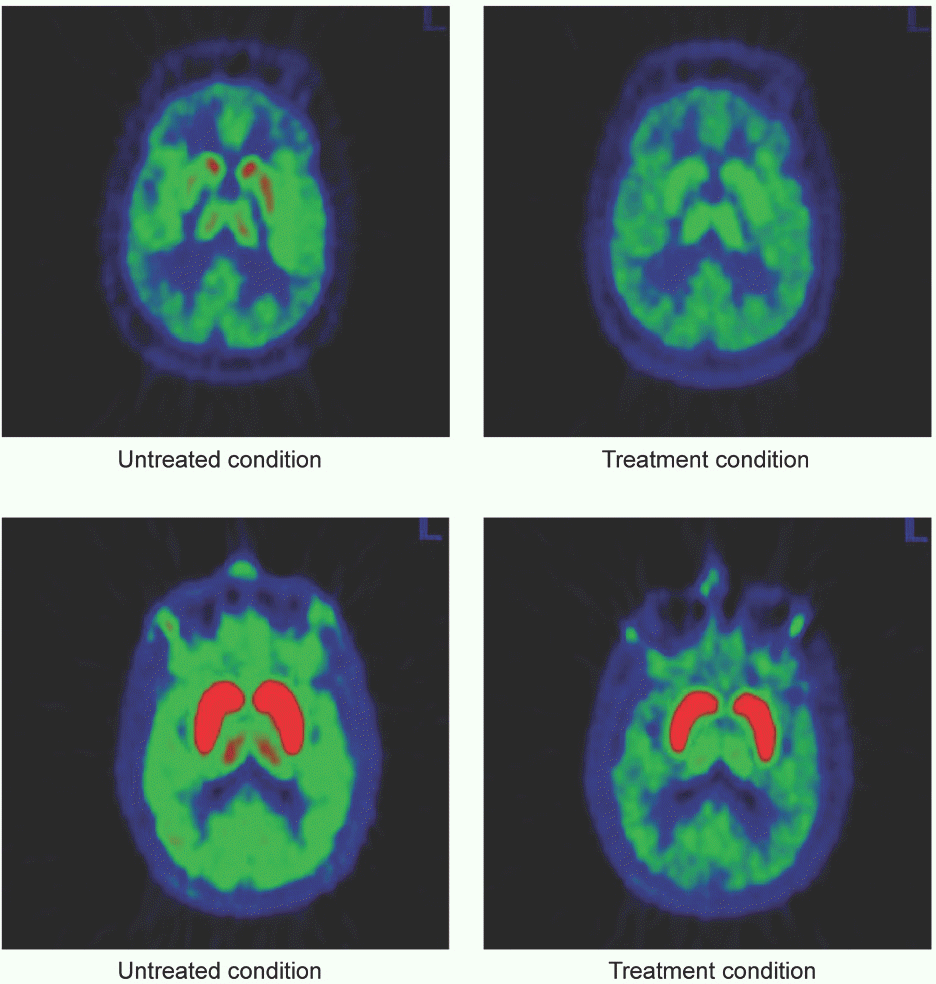 | Figure 1.Occupancy effect of SKL10406 upon [11C] DASB (top) and [11C] PE2I (bottom) brain uptake |
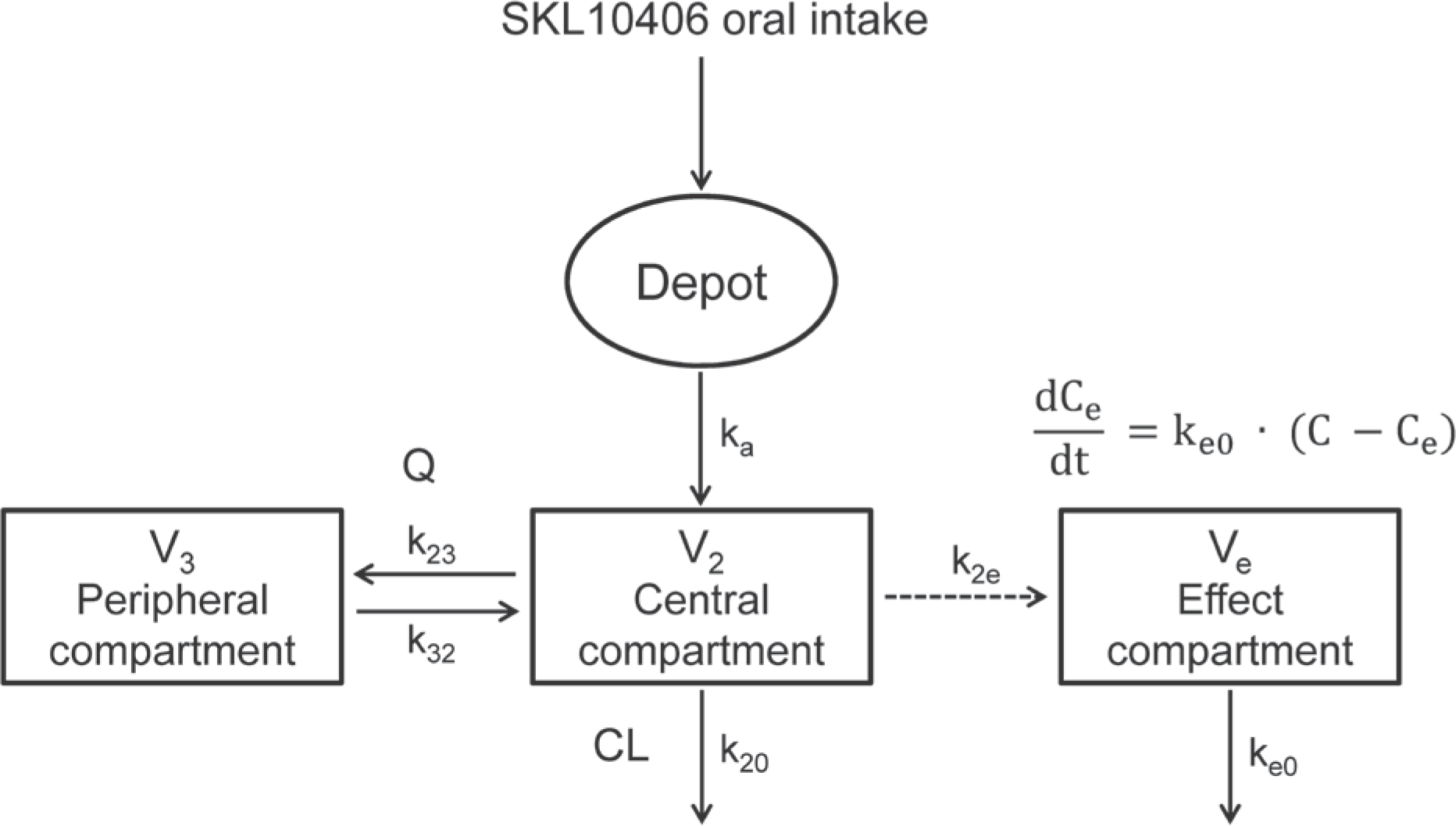 | Figure 2.Effect compartmental PK-PD model (EME) for SKL10406. C, drug concentration in the central compartment; Ce, drug concentration in the effect compartment; CL, clearance; ka, absorption rate constant; Ke0, equilibrium rate constant; Q, inter-compartmental clearance |
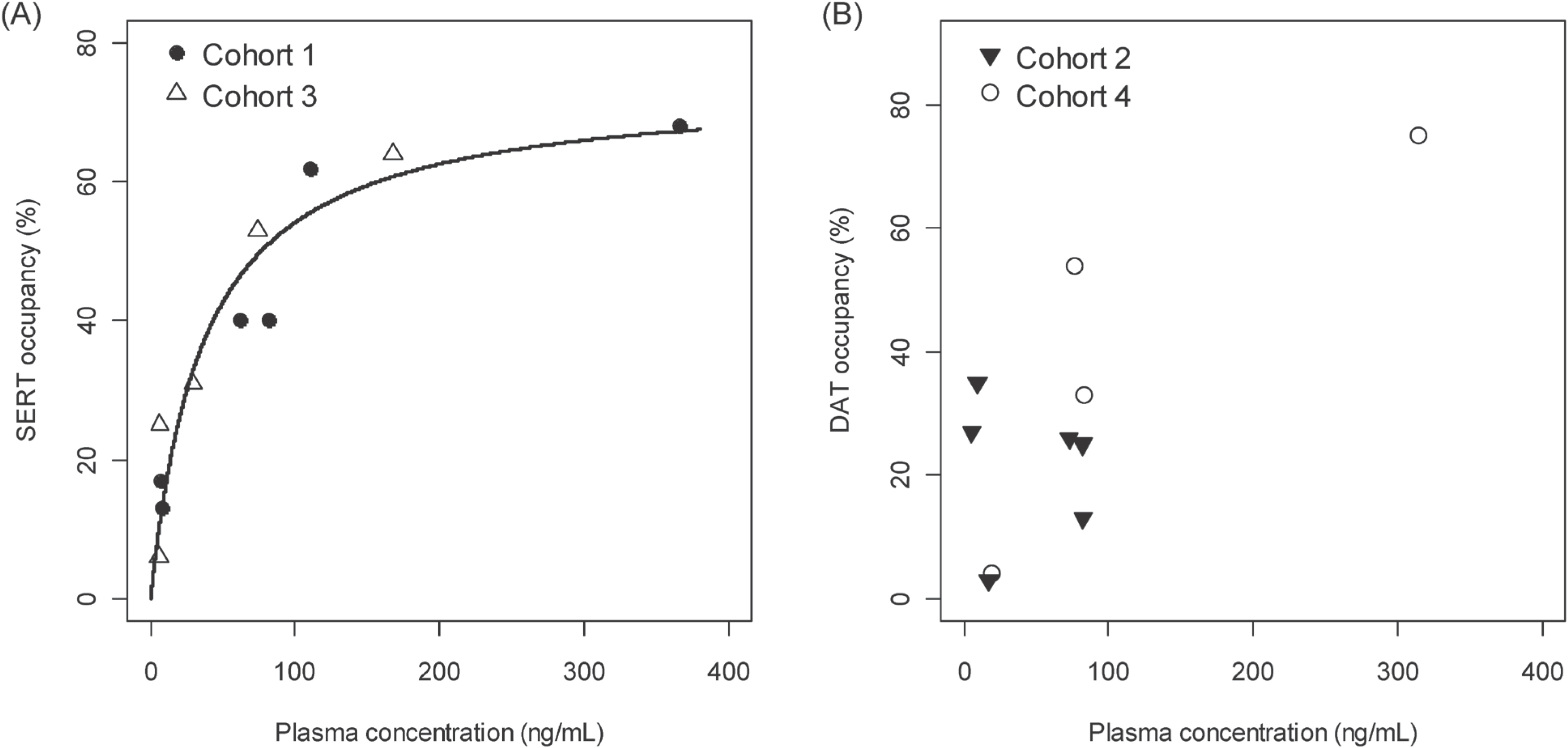 | Figure 3.Plasma concentration and striatal SERT (panel A) and DAT (panel B) occupancy at 4 hours and 16 hours after dosing. The X-axis represents the plasma concentration of SKL10406 in ng/mL and the Y-axis represents the percent occupancy of serotonin/dopamine transporters in the striatum. The regression curve in the left panel (A) was obtained from NPM results. |
 | Figure 4.Visual predictive check plots of the final pharmacokinetic and pharmacodynamic models. 1,000 data sets were simulated using the parameter estimates of the final model. Prediction interval curves of the 12.5th, 50th, and 87.5th percentiles from 1,000 simulated datasets from the PK and PD models were overlaid with observed data. Vertical dashed lines indicate the Tmax of SKL10406 and are shown to demonstrate a delay in predicted occupancy changes by EME compared with DME. |
Table 3.
Final model estimates of population pharmacokinetic and pharmacodynamic parameters
| Parameter | Typical value | IIV (%)a | RSE (%)b | Bootstrap median (95% CI)c |
|---|---|---|---|---|
| Pharmacokinetic parameters (ME) | ||||
| CL (L/h) | 49.6 | 62.2 | 19.0 | 43.7 (32.9–68.9) |
| V2 (L) | 176 | 41.6 | 14.4 | 169 (15.9–221) |
| ka (h−1) | 5.05 | 57.6 | 18.6 (0.51–32.4) | |
| V3 (L) | 63.7 | 33.2 | 51.2 (38.8–133) | |
| Q (L/h) | 21.1 | 18.3 | 19.0 (15.0–32.8) | |
| ALAG (h) | 0.336 | 27.0 | 0.429 (0.18–0.48) | |
| Pharmacodynamic parameters (NPM) | ||||
| Emax (%) | 74.1 | 7.71 | ||
| EC50 (ng/mL) | 36.8 | 31.3 | ||
| Pharmacodynamic parameters (DME) | ||||
| Emax (%) | 53.4 | 20.7 | 8.31 | 53.1 (44.2–65.9) |
| EC50 (ng/mL) | 11.8 | 44.9 | 23.6 | 12.1 (7.23–28.5) |
| Pharmacodynamic parameters (EME) | ||||
| Emax (%) | 68.6 | 4.62 | 69.9 (50.3–104.8) | |
| EC50 (ng/mL) | 40.2 | 68.3 | 34.3 | 44.2 (12.9–140) |
| Ke0 (h−1) | 0.288 | 9.65 | 0.286 (0.202–0.409) | |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download