Abstract
A simple, rapid and selective liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) is developed and validated for quantification of venlafaxine in human plasma with simple liquid-liquid extraction step consisted of extraction with ether and dichloromethane for 10 min and mixing with 1 M sodium acetate in human plasma using fluoxetine as an internal standard (IS). The analyte are separated using an isocratic mobile phase consisted of acetonitrile and 5 mM ammonium formate (4/3, v/v) on a isocratic YMC hydrosphere C18 (2.0x50.0 mm, 3.0 μm) column and analyzed by MS/MS in the multiple reaction monitoring (MRM) mode using the transitions of respective [M+H]+ ions, m/z 278.2→260.3 and m/z 310.1→148.1 for quantification of venlafaxine and IS, respectively. The standard calibration curves showed good linearity within the range of 1.0–200.0 ng/mL (r2=0.9986, 1/x2 weighting). The lower limit of quantification (LLOQ) was 1.0 ng/mL. The retention times of venlafaxine and IS were 0.6 min and 0.7 min that means the potential for the high-throughput potential of the proposed method. In addition, no significant metabolic compounds were found to interfere with the analysis. Acceptable precision and accuracy were obtained for the concentrations over the standard curve range. The validated method was successfully applied to bioequivalence study after 75-mg of venlafaxine sustained-release (SR) capsule in 24 healthy Korean subjects.
References
1. Muth EA, Moyer JA, Haskins JT. Biochemical, neurophysiological, and behavioral effects of WY-45, 233 and other identified metabolites of the antidepressant venlafaxine. Drug Dev Res. 1991; 23:191–199.
2. Rudorfer MV, Potter WZ. Antidepressants. A comparative review of the clinical pharmacology and therapeutic use of the ‘newer' versus the ‘older' drugs. Drugs. 1989; 37:713–738.
3. Andrews JM, Ninan PT, Nemeroff CB. Venlafaxine: a novel antidepressant that has a dual mechanism of action. Depression. 1996; 4:48–56.

4. Nowakowska E, Kus K, Chodera A. Comparison of behavioural effects of venlafaxine and imipramine in rats. Arzneimittelforschung. 2003; 53:237–242.

5. Czubak A, Nowakowska E, Kus K, Matschay A, Kokot Z. The effects of nicotine and mecamylamine on spatial memory in rats. Eur Neuropsychopharmacol. 2007; 17:253–254.
6. Tizabi Y, Rezvani AH, Russell LT, Tyler KY, Overstreet DH. Depressive characteristics of FSL rats: involvement of central nicotinic receptors. Pharmacol Biochem Behav. 2000; 66:73–77.
7. Hicks DR, Wolaniuk D, Russell A, Cavanaugh N, Kraml M. A high-performance liquid chromatographic method for the simultaneous determination of venlafaxine and O-desmethylvenlafaxine in biological fluids. Ther Drug Monit. 1994; 16:100–107.

8. Deecher DC, Beyer CE, Johnston G, Bray J, Shah S, Abou-Gharbia M. Desvenlafaxine succinate: A new serotonin and norepinephrine reuptake inhibitor. J Pharmacol Exp Ther. 2006; 318:657–665.

9. Cherkaoui S, Rudaz S, Varesio E, Veuthey JL. On-line capillary electrophoresis-electrospray mass spectrometry for the stereoselective analysis of drugs and metabolites. Electrophoresis. 2001; 22:3308–3315.

10. Rudaz S, Stella C, Balant-Gorgia AE, Fanali S, Veuthey JL. Simultaneous stereoselective analysis of venlafaxine and O-desmethylvenlafaxine enantiomers in clinical samples by capillary electrophoresis using charged cyclodextrins. J Pharm Biomed Anal. 2000; 23:107–115.

11. Papoutsis I, Khraiwesh A, Nikolaou P, Pistos C, Spiliopoulou C, Athanas-elis S. A fully validated method for the simultaneous determination of 11 antidepressant drugs in whole blood by gas chromatography-mass spectrometry. J Pharm Biomed Anal. 2012; 70:557–562.

12. Vu RL, Helmeste D, Albers L, Reist C. Rapid determination of venlafaxine and O-desmethylvenlafaxine in human plasma by high-performance liquid chromatography with fluorimetric detection. J Chromatogr B Biomed Sci Appl. 1997; 703:195–201.

13. Bhatt J, Jangid A, Venkatesh G, Subbaiah G, Singh S. Liquid chromatography-tandem mass spectrometry (LC-MS-MS) method for simultaneous determination of venlafaxine and its active metabolite O-desmethyl venlafaxine in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2005; 829:75–81.

14. Wei Z, Bing-Ren X, Cai-Yun W. Liquid chromatography-mass spectrometry method for the determination of venlafaxine in human plasma and application to a pharmacokinetic study. Biomed Chromatogr. 2007; 21:266–272.

15. Patel BN, Sharma N, Sanyal M, Shrivastav PS. Liquid chromatography tandem mass spectrometry assay for the simultaneous determination of venlafaxine and O-desmethylvenlafaxine in human plasma and its application to a bioequivalence study. J Pharm Biomed Anal. 2008; 47:603–611.

16. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinfor-mation/guidances/ucm070107.pdf.(accessedMay12,2014)
17. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatory-Information/Guidances/UCM377465.pdf.(accessedMay12,2014)
19. Farrier DS. PK Solutions; Pharmacokinetics Data Analysis User Guide. Version 2.0. Summit Research Services, Montrose, Colorado. 2005.
20. Wright CW, Aikman MS, Werts E, Seabolt J, Haeusler JM. Bioequivalence of single and multiple doses of venlafaxine extended-release tablets and capsules in the fasted and fed states: four open-label, randomized crossover trials in healthy volunteers. Clin Ther. 2009; 31:2722–2734.

21. Troy SM, Dilea C, Martin PT, Leister CA, Fruncillo RJ, Chiang ST. Pharmacokinetics of once-daily venlafaxine extended release in healthy volunteers. Curr Ther Res Clin Exp. 1997; 58:504–514.

22. Effexor XR. Venlafaxine hydrochloride. Extended-release capsules [package insert] Wyeth Pharmaceuticals Inc, Philadelphia. 2009.
Figure 1.
Chemical structures of (A) venlafaxine [(RS)-1-[2-dimethylamino-1-(4-methoxyphenyl)-ethyl]-cyclohexanol, MW=277.402 g/ mol, C17H27NO2] and (B) fluoxetine [IS, (RS)-N-methyl-3-phenyl-3-[4-(trifluoromethyl) phenoxy] propan-1-amine, MW=309.33 g/mol, C17H18F3NO] (from ChemSpider free chemical data base).
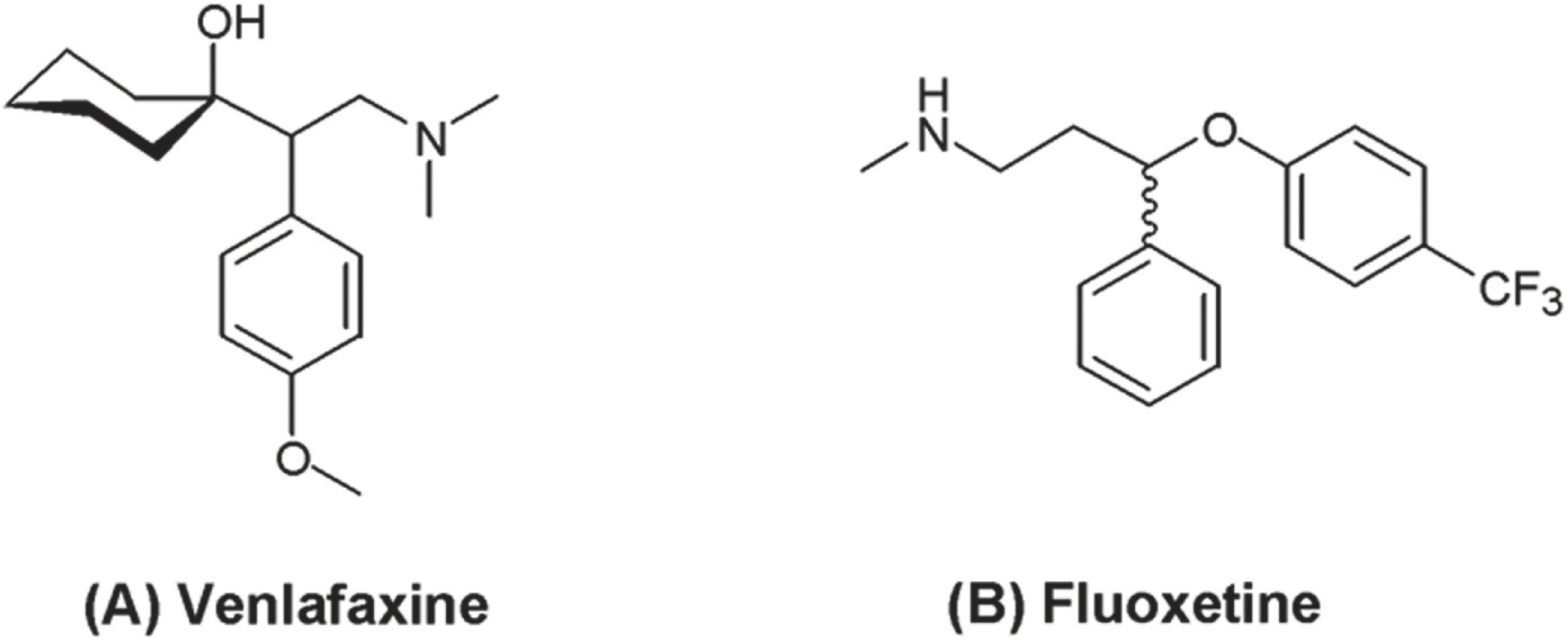
Figure 2.
Chromatograms of (A) double blank plasma without venlafaxine and IS, (B) with IS (10.0 μg/mL), (C) with venlafaxine (LLOQ, 1.0 ng/mL) and IS (10.0 μg/mL), and (D) subject's plasma taken 10.0 h after a single oral dose of 75-mg venlafaxine capsule spiked with IS (10.0 μg/mL).
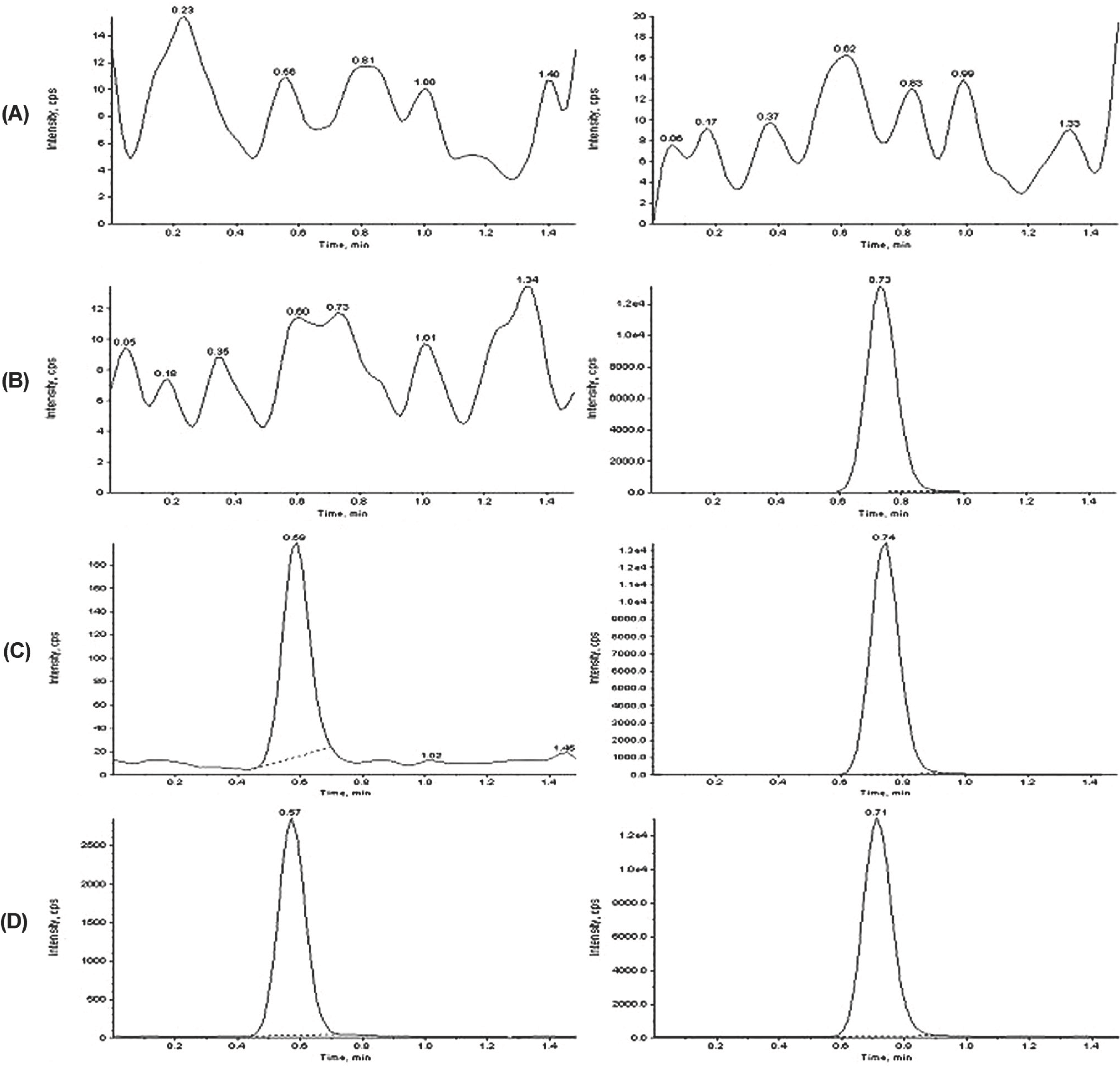
Figure 3.
Full-scan mass spectra of precursor (A, C) and product ions (B, D) of venlafaxine (m/z 278.2→260.4) and fluoxetine (m/z 310.2→148.1), respectively.
Figure 4.
Mean plasma concentrations versus time plots after a single oral dose of 75-mg venlafaxine SR capsule to the 24 healthy male subjects (○: reference, ●: test) (n=24, Mean±SD).
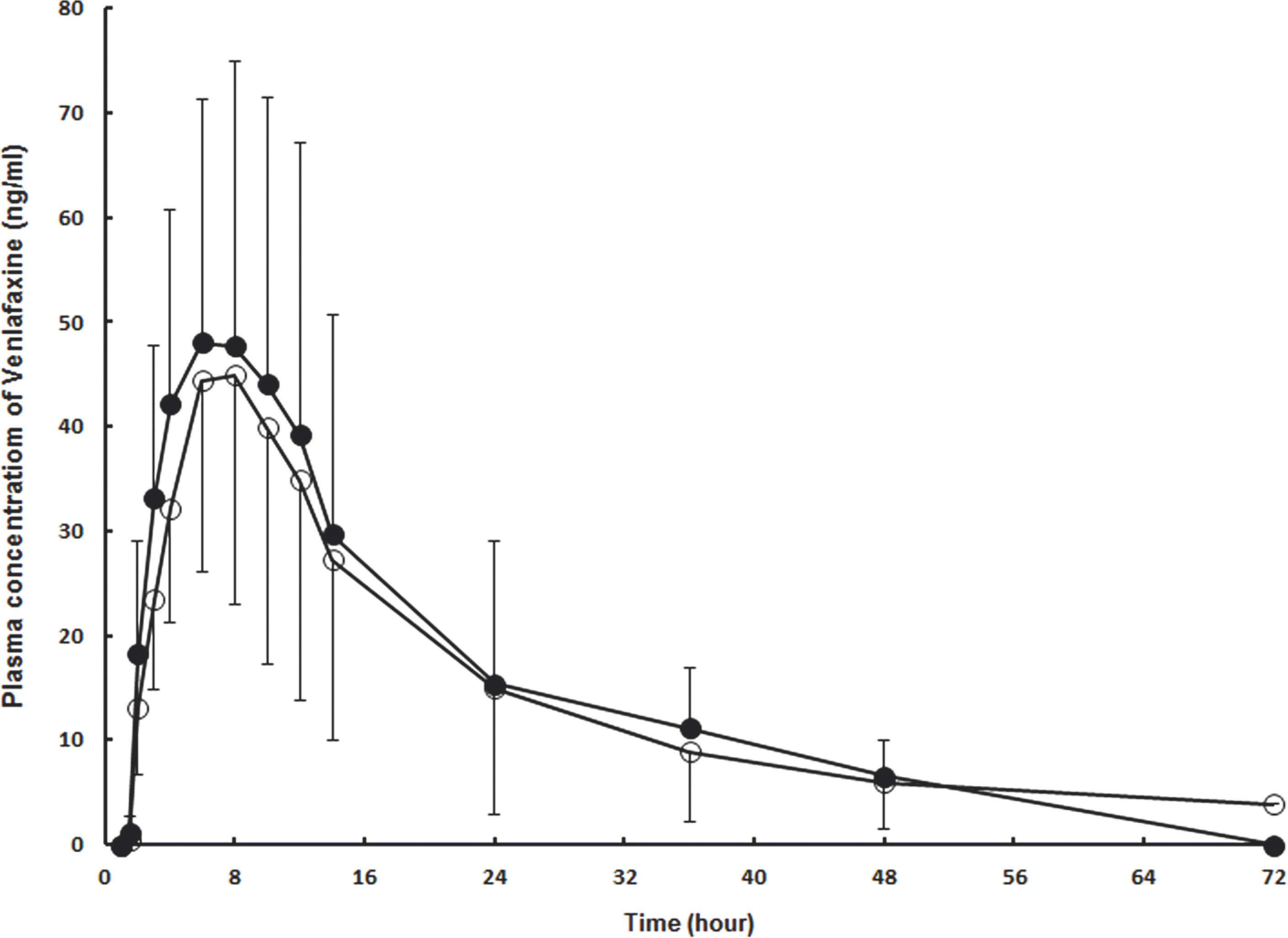
Table 1.
Method validation for the analysis of venlafaxine in human plasma and recovery of venlafaxine and IS (fluoxetine) after the extraction pro cedure, n=5
| Nominal Concentration (ng/mL) | Precision (%RSD)b | Accuracy (%) | ||
|---|---|---|---|---|
| Inter-day | Intra-day | Inter-day | Intra-day | |
| 1 (LLOQa) | 11.87 | 5.26 | 94.30 | 105.04 |
| 6 (low) | 5.04 | 5.03 | 102.39 | 101.02 |
| 100 (middle) | 4.38 | 2.55 | 101.94 | 106.27 |
| 160 (high) | 5.10 | 1.80 | 95.22 | 97.94 |
Table 2.
Pharmacokinetic parameters (mean±SD, n=24) of two formulations of 75-mg venlafaxine SR capsule based on its blood concentrations in the 24 healthy subjects
AUCt=area under plasma concentration-time curve from time 0 to t; AUC∞=area under plasma concentration-time curve from time 0 to infinite time; AUMCt=area under first moment of plasma concentration-time curve from time 0 to t; AUMC∞=area under first moment of plasma concentration-time curve from time 0 to infinite time; Vd=apparent volume of distribution; MRT=mean residence time; Cmax= peak plasma concentration; T1/2α=absorption half-life; Tmax=time for the Cmax; ka=absorption rate constant; T1/2α=elimination half-life; λz(ke) =elimination rate constant; CL=clearance.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download