Abstract
Ibandronate (a bisphosphonate) is commonly used as an treatment of osteoporosis in combination with vitamin D. Monthly DP-R206-a novel, fixed-dose combination tablet (150 mg ibandro-nate/24,000 IU vitamin D3)-was recently developed to enhance patient compliance. This open, randomized, two-period crossover study was conducted to compare the pharmacokinetics of ibandronate when administered as DP-R206 or 150 mg ibandronate to healthy adult volunteers. Each volunteer was randomly allocated to receive single-dose DP-R206 or ibandronate with a 28-day washout period between treatments. Blood samples were assessed using pharmacokinetic analysis. Plasma ibandronate concentrations were determined using liquid chromatography-tandem mass spectrometry. Safety and tolerability assessments were performed throughout the study. In total, 103 participants received the study drugs and 72 participants completed the study. The geometric mean ratios (DP-R206/ibandronate) of the maximum concentration (Cmax) and the area under the plasma concentration time curve from time zero to the last concentration (AUClast) values were 0.959 (90% CI: 0.820–1.120) and 0.924 (90% CI: 0.805–1.060), respectively. The frequencies of adverse events (AEs) and drug reactions were similar between treatment groups, and all AEs were recovered without sequalae. Ibandronate pharmacokinetics, tolerability, and safety are comparable when administered to healthy individuals, regardless if administered as DP-R206 or ibandronate.
References
1. Luckman SP, Hughes DE, Coxon FP, Graham R, Russell G, Rogers MJ. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J Bone Miner Res. 1998; 13:581–589.

2. Cremers SC, Pillai G, Papapoulos SE. Pharmacokinetics/pharmacody-namics of bisphosphonates: use for optimisation of intermittent therapy for osteoporosis. Clin Pharmacokinet. 2005; 44:551–570.
3. Clinician's guide to prevention and treatment of osteoporosis. 2014. http://nof.org/hcp/clinicians-guide/Accessed17Feb.
4. Penning-van Beest FJ, Goettsch WG, Erkens JA, Herings RM. Determinants of persistence with bisphosphonates: a study in women with postmenopausal osteoporosis. Clin Ther. 2006; 28:236–242.

5. Mühlbauer RC, Bauss F, Schenk R, Janner M, Bosies E, Strein K, et al. BM 21.0955, a potent new bisphosphonate to inhibit bone resorption. J Bone Miner Res. 1991; 6:1003–1011.

6. Lewiecki EM, Babbitt AM, Piziak VK, Ozturk ZE, Bone HG. Adherence to and gastrointestinal tolerability of monthly oral or quarterly intravenous ibandronate therapy in women with previous intolerance to oral bisphosphonates: a 12-month, open-label, prospective evaluation. Clin Ther. 2008; 30:605–621.

7. Reginster JY, Felsenberg D, Cooper C, Stakkestad JA, Miller PD, Kendler DL, et al. A new concept for bisphosphonate therapy: a rationale for the development of monthly oral dosing of ibandronate. Osteoporos Int. 2006; 17:159–166.

8. Busse B, Bale HA, Zimmermann EA, Panganiban B, Barth HD, Carriero A, et al. Vitamin D deficiency induces early signs of aging in human bone, increasing the risk of fracture. Sci Transl Med. 2013; 5:193ra88. doi: 10.1126/scitranslmed.3006286.

9. Bischoff-Ferrari HA, Willett WC, Orav EJ, Lips P, Meunier PJ, Lyons RA, et al. A pooled analysis of vitamin D dose requirements for fracture prevention. N Engl J Med. 2012; 367:40–49. doi: 10.1056/NEJMoa1109617.

10. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Guidelines for preventing and treating vitamin D deficiency and insufficiency revisited. J Clin Endocrinol Metab. 2012; 97:1153–1158. doi: 10.1210/jc.2011-2601.

11. Sohl E, van Schoor NM, de Jongh RT, Visser M, Deeg DJ, Lips P. Vitamin D status is associated with functional limitations and functional decline in older individuals. J Clin Endocrinol Metab. 2013; 98:E1483–E1490. doi: 10.1210/jc.2013-1698.

12. Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, Orav JE, Stuck AE, Theiler R, et al. Fall prevention with supplemental and active forms of vitamin D: a metaanalysis of randomised controlled trials. BMJ. 2009; 339:b3692. doi: 10.1136/bmj.b3692.

13. MacLaughlin J, Holick MF. Aging decreases the capacity of human skin to produce vitamin D3. J Clin Invest. 1985; 76:1536–1538.

14. Holick MF, Matsuoka LY, Wortsman J. Age, vitamin D, and solar ultraviolet. Lancet. 1989; 2:1104–1105.

15. Mosali P, Bernard L, Wajed J, Mohamed Z, Ewang M, Moore A, et al. Vitamin D status and parathyroid hormone concentrations influence the skeletal response to zoledronate and denosumab. Calcif Tissue Int. 2014; 94:553–559. doi: 10.1007/s00223-014-9840-0.

16. Deane A, Constancio L, Fogelman I, Hampson G. The impact of vitamin D status on changes in bone mineral density during treatment with bisphosphonates and after discontinuation following longterm use in postmenopausal osteoporosis. BMC Musculoskelet Disord. 2007; 8:3.

17. Reginster JY, Burlet N. Osteoporosis: a still increasing prevalence. Bone. 2006; 38(2 Suppl 1):S4–S9.

18. Choi HJ, Shin CS, Ha YC, Jang S, Jang S, Park C, et al. Burden of osteoporosis in adults in Korea: a national health insurance database study. J Bone Miner Metab. 2012; 30:54–58. doi: 10.1007/s00774-011-0280-x.

19. Bonviva (ibandronate sodium) tablets. http://www.accessdata.fda.gov/drugsatfda_docs/label/2005/021455s001lbl.pdf. Accessed 17 Feb. 2014.
20. Miller PD, McClung MR, Macovei L, Stakkestad JA, Luckey M, Bonvoisin B, et al. Monthly oral ibandronate therapy in postmenopausal osteoporosis: 1-year results from the MOBILE study. J Bone Miner Res. 2005; 20:1315–1322.

21. Miller PD, Epstein S, Sedarati F, Reginster JY. Once-monthly oral ibandronate compared with weekly oral alendronate in postmenopausal osteoporosis: results from the head-to-head MOTION study. Curr Med Res Opin. 2008; 24:207–213.

22. Barrett J, Worth E, Bauss F, Epstein S. Ibandronate: a clinical pharmacological and pharmacokinetic update. J Clin Pharmacol. 2004; 44:951–965.

23. Diletti E, Hauschke D, Steinijans VW. Sample size determination for bioequivalence assessment by means of confidence intervals. Int J Clin Pharmacol Ther Toxicol. 1991; 29:1–8.
24. Jeon JY, Lee SY, Im YJ, Kim EY, Kim Y, Park TS, et al. Comparison of the pharmacokinetics, safety, and tolerability of vitamin D3 in DP-R206 (150-mg ibandronate/24,000-IU vitamin D3 tablet) and as monotherapy (24,000 IU) in healthy male Korean adults. Clin Ther. 2014; 36:48–57. doi: 10.1016/j. clinthera.2013.12.001.

Figure 2.
Mean plasma ibandronate concentration-time curves (semilog (upper) and linear (lower) scale)
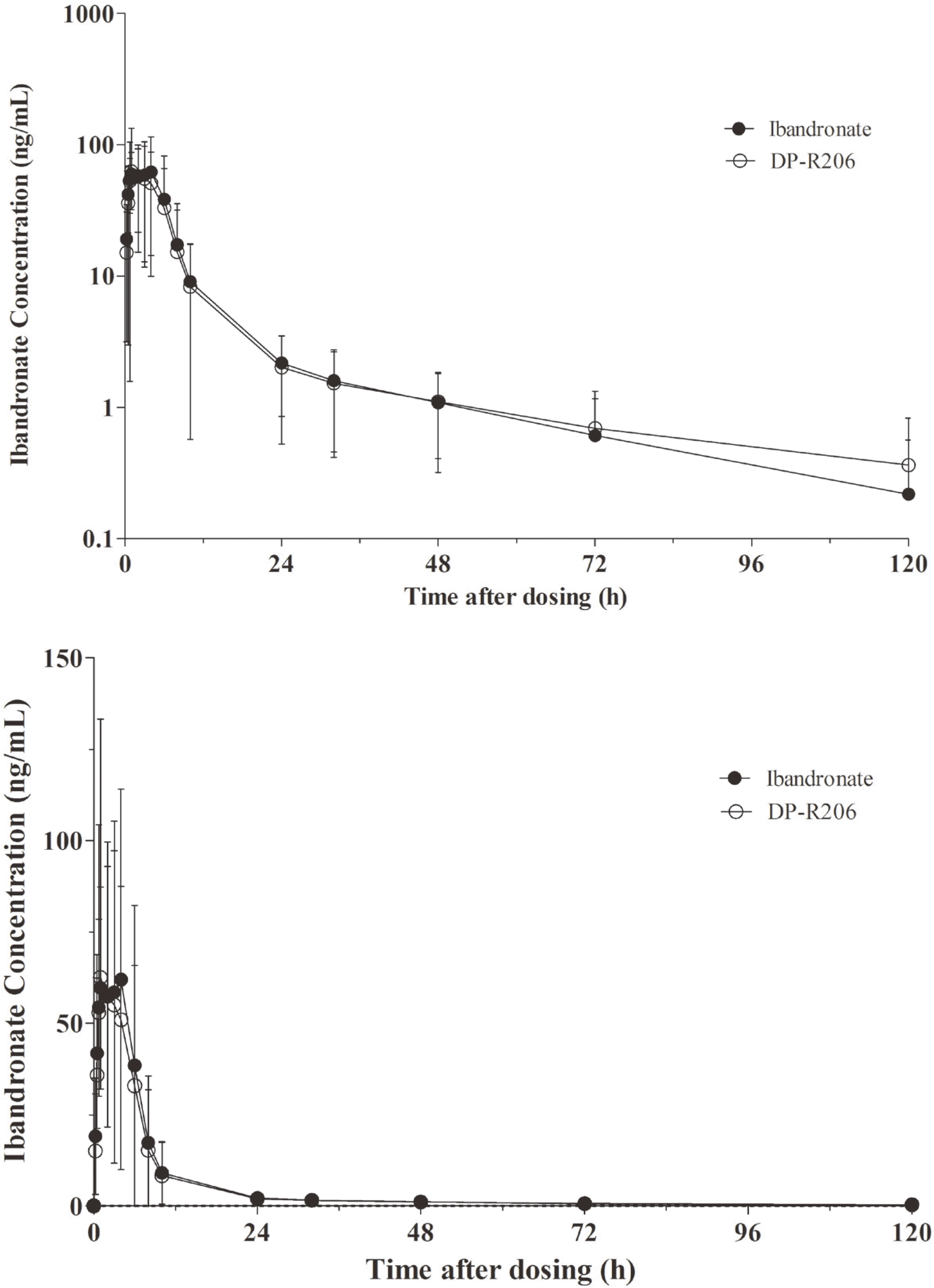
Table 1.
Demographic characteristics of the enrolled subjects
| RT (n=49) | TR (n=54) | Total (n=103) | p | ||
|---|---|---|---|---|---|
| Age (yr) | 24.71±2.75 | 24.70±3.49 | 24.71±3.15 | 0.694∗¶ | |
| Weight (kg) | 70.24±8.73 | 71.71±8.03 | 71.01±8.37 | 0.579†¶ | |
| Height (cm) | 174.62±4.92 | 175.15±4.59 | 174.90±4.73 | 0.376†¶ | |
| Drinking‡ | Yes | 63.3% (31) | 61.1% (33) | 62.1% (64) | 0.822§ |
| No | 36.7% (18) | 38.9% (21) | 37.9% (39) | ||
| Smoking‡ | Yes | 38.8% (19) | 33.3% (18) | 35.9% (37) | 0.565§ |
| No | 61.2% (30) | 66.7% (36) | 64.1% (66) | ||
| Caffeine‡ | Yes | 61.2% (30) | 55.6% (30) | 58.3% (60) | 0.560§ |
| No | 38.8% (19) | 44.4% (24) | 41.7% (43) | ||
| Subjects who administered the study drug at least once were included in this analysis. | |||||
Table 2.
Pharmacokinetic parameters
| PK parameter | Summary statistics | Ibandronate (150 mg) | DP-R206∗ |
|---|---|---|---|
| Cmax (ng/mL) |
Arithmetic mean SD CV (%) |
90.64 54.72 60.4 |
87.70 74.52 85.0 |
| AUClast (ng/mL) |
Arithmetic mean SD CV (%) |
530.66 340.96 65.9 |
482.80 325.34 67.4 |
| AUCinf (ng·hr/mL) |
Arithmetic mean SD CV (%) |
549.24 366.76 66.8 |
501.43 349.59 69.7 |
| Tmax (hr) |
Median Minimum Maximum |
2.00 0.50 6.00 |
2.00 0.50 6.00 |
| t1/2 (hr) |
Mean SD CV (%) |
32.38 15.29 47.21 |
30.76 16.52 53.71 |
Table 3.
Geometric mean ratio and 90% confidence interval limits of the primary ibandronate PK parameters (DP-R206∗:150 mg tablet ibandronate)
| PK parameter | Geometric mean ratio | 90% Confidence interval |
|---|---|---|
| Cmax | 0.959 | 0.820–1.120 |
| AUClast | 0.924 | 0.805–1.060 |
| AUCinf | 0.923 | 0.801–1.062 |
Table 4.
Adverse events
| Treatment | Ibandronate 150 mg (n=85) | DP-R206∗ (n=90) | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| System organ class | Severity | Probably related | Possibly related | Unlikely related | Definitely not related | Probably related | Possibly related | Unlikely related | Definitely not related | |
| Gastrointestinal disorders | Mild | 27 | 6 | 0 | 0 | 35 | 7 | 0 | 0 | 75 |
| Moderate | 2 | 1 | 0 | 0 | 6 | 2 | 0 | 0 | 11 | |
| Severe | 1 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 4 | |
| General disorders and administration site conditions | Mild | 19 | 1 | 0 | 0 | 20 | 1 | 0 | 0 | 41 |
| Moderate | 7 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 13 | |
| Severe | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | |
| Immune system disorders | Mild | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Moderate | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Severe | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |
| Infections and infestations | Mild | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 2 |
| Moderate | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Severe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Injury, poisoning, and procedural complications | Mild | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Moderate | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Severe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Investigations | Mild | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 3 |
| Moderate | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Severe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Metabolic and nutritional disorders | Mild | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| Moderate | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Severe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Musculoskeletal and connective tissue disorders | Mild | 25 | 0 | 0 | 0 | 26 | 1 | 0 | 0 | 52 |
| Moderate | 8 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 14 | |
| Severe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Nervous system disorders | Mild | 9 | 7 | 0 | 0 | 9 | 3 | 0 | 1 | 29 |
| Moderate | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | |
| Severe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Renal and urinary disorders | Mild | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Moderate | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Severe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Respiratory, thoracic, and mediastinal disorders | Mild | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 3 | 5 |
| Moderate | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Severe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Dermal and subcutaneous tissue disorders | Mild | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Moderate | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Severe | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |
| Total | 99 | 21 | 3 | 0 | 112 | 16 | 2 | 5 | 258 | |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download