Abstract
Levofloxacin is a bactericidal broad spectrum antibiotic against Gram-positive and Gram-negative pathogens. A randomized, two-treatment, two-period, two-way crossover study was conducted to evaluate the bioequivalence of Lectacin 250 mg tablet, a generic levofloxacin, to its reference drug, Cravit 250 mg tablet. Each period was separated by a 7-day washout. Serial blood samples were collected until 24 h after dosing and plasma levofloxacin concentrations were determined using a high performance liquid chromatography. Pharmacokinetic parameters were analyzed using K-BE Test 2007 and BA calc 2007 (Ministry of Food and Drug Safety, Cheongju-si, South Korea). The peak concentration (Cmax) and the area under the plasma concentration versus time curve from 0 to the last measurable concentration (AUC0-t) for the generic and reference levofloxacin were 4.48±0.89 mg/L and 4.46± 0.95 mg/L, and 25.33±4.12 mg∗h/L and 25.77±4.01 mg∗h/L, respectively, leading to a geometric mean ratio (90% confidence interval) of the generic to the reference levofloxacin of 1.0060 (0.9339–1.0842) and 0.9810 (0.9476–1.0159), respectively, for Cmax and AUC0-t. Lectacin 250 mg tablet is bioequivalent to Cravit 250 mg tablet.
References
1. North DS, Fish DN, Redington JJ. Levofloxacin, a second-generation fluoroquinolone. Pharmacotherapy. 1998; 18:915–935.
2. Croom KF, Goa KL. Levofloxacin: a review of its use in the treatment of bacterial infections in the United States. Drugs. 2003; 63:2769–2802.
3. Rafat C, Debrix I, Hertig A. Levofloxacin for the treatment of pyelonephritis. Expert Opin Pharmacother. 2013; 14:1241–1253. doi: 10.1517/14656566. 2013.792805.

4. Torres A, Liapikou A. Levofloxacin for the treatment of respiratory tract infections. Expert Opin Pharmacother. 2012; 13:1203–1212. doi: 10.1517/14 656566.2012.688952.

5. Anderson VR, Perry CM. Levofloxacin: a review of its use as a high-dose, short-course treatment for bacterial infection. Drugs. 2008; 68:535–565.
6. Guidance Document for Bioequivalence Study. In: MFDS, editor. 2010.
7. Tsaganos T, Kouki P, Digenis P, Giamarellou H, Giamarellos-Bourboulis EJ, Kanellakopoulou K. Pharmacokinetics of levofloxacin after single and multiple oral doses in patients undergoing intermittent haemodialysis. Int J Antimicrob Agents. 2008; 32:46–49. doi: 10.1016/j.ijantimicag.2008.02.011.

8. Levaquin® (levofloxacin tablets, oral solution, injection): US prescribing information. In: Ortho-McNeil Pharmaceutical I, editor. 2008.
9. Chien SC, Rogge MC, Gisclon LG, Curtin C, Wong F, Natarajan J, et al. Pharmacokinetic profile of levofloxacin following once-daily 500-milligram oral or intravenous doses. Antimicrob Agents Chemother. 1997; 41:2256–2260.

10. Gao X, Yao G, Guo N, An F, Guo X. A simple and rapid high performance liquid chromatography method to determine levofloxacin in human plasma and its use in a bioequivalence study. Drug Discov Ther. 2007; 1:136–140.
Figure 1.
Mean plasma concentration – time curves of levofloxacin after a single oral dose of levofloxacin 250 mg. The error bars represent the standard deviations (Upper: linear scale, Lower: log scale)
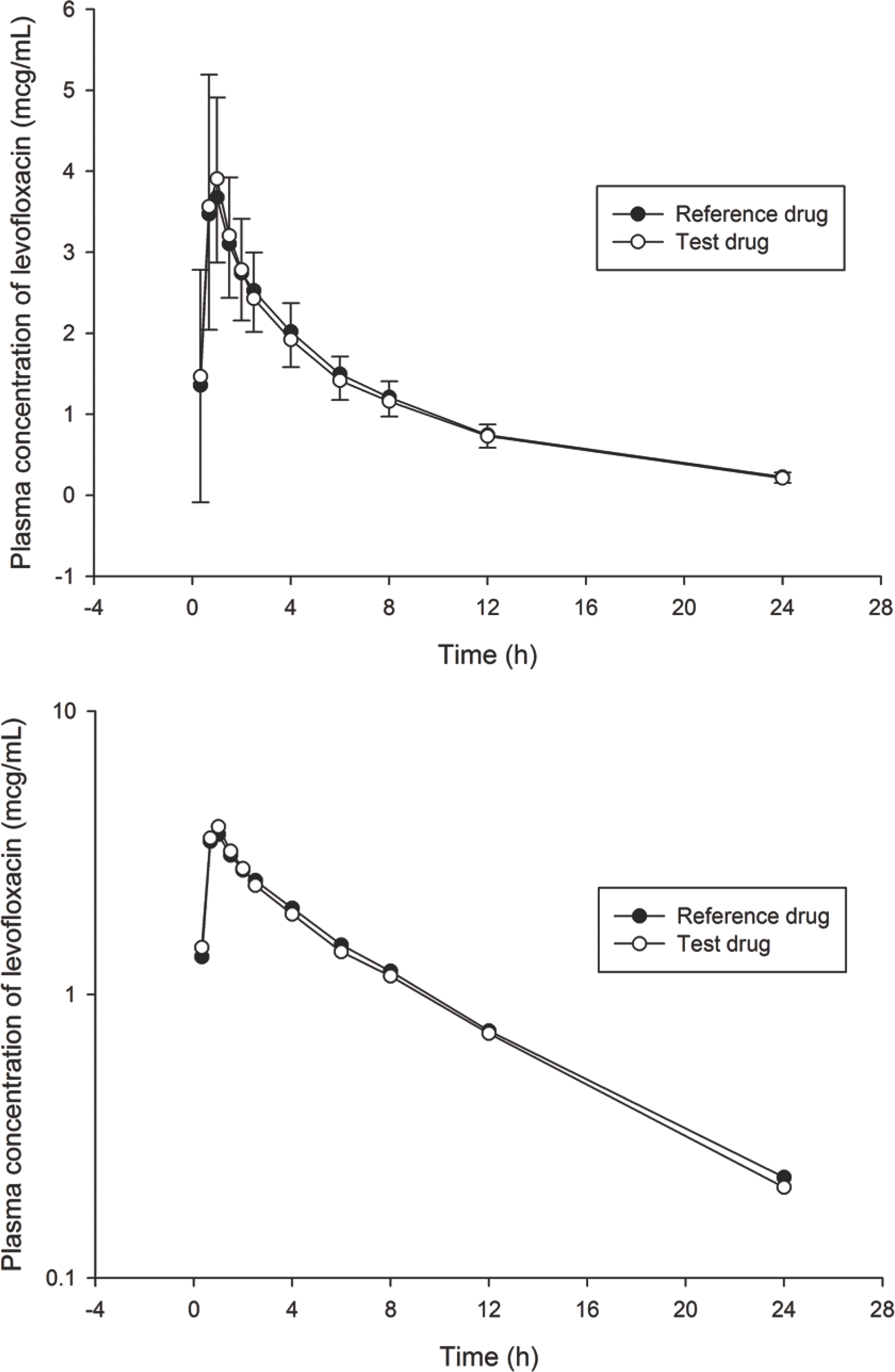
Table 1.
Accuracy and precision of levofloxacin concentration measurement by LC-MS/MS method (n=5)
Table 2.
Demographic characteristics of the volunteers
| Characteristics | Values |
|---|---|
| Age (years) | 24.0±2.2 |
| Height (cm) | 176.8±5.1 |
| Body weight (kg) | 70.2±8.6 |
Table 3.
Pharmacokinetic parameters of levofloxacin after a single oral dose administration of levofloxacin at 250 mg
All values are presented as mean±SD [geometric mean], except for tmax, for which median (range) is presented. AUC0-t = area under the plasma concentration-time curve (AUC) from time 0 to 24 hours post dose; Cmax = peak plasma drug concentration; tmax = time to reach peak plasma concentration; Test = Lectacin 250 mg tablet; Reference = Cravit 250 mg tablet




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download