INTRODUCTION
The human masticatory system produces complicated motions during ingestion, swallowing, and pronunciation. In particular, various masticatory muscles perform an intricate lateral movement of the mandible while chewing food. The distribution of stress in and distortion of the mandible during lateral movements are known to be closely linked to bruxism, dental implant placement, and temporomandibular joint (TMJ) disorder [
1234].
Comprehensive study of the phenomenon that is mastication has been achieved by means of cadaveric dissection, reading electromyography (EMG) recordings, functional magnetic resonance imaging (fMRI), motion detection analysis, finite element (FE) modeling, and clinical observation [
15678910]. More specifically, researchers have employed FE modeling and quantitative analysis in investigations of stress and strain distributions in the mandible and in the TMJ [
911]. The location (origin and insertion) and dimensions (length and volume) of the masticatory muscles are typically regarded as force factors in simulations of masticatory cycles in FE studies. The function of these muscles is known to be more complicated than presumed by this simplification and its theoretically postulated contraction forces and dimensional muscle changes [
1011]. It seems more prudent to make use of the path traced by the incisors of a living subject during mastication. This description of the chewing motion of the mandible represents a realized outcome, rendered by the actual interaction of the complicated muscular vectors. Consequently, we took this approach in our study.
The study promises to have clinical, in addition to technical, implications. The object of our FE analysis, stress distribution and distortion of the mandible according to the anteroposterior intermaxillary relationship, is important for clinical reasons. Tooth size disproportion can be used to predict stress distribution and distortion in TMJ disorder, bruxism, and occlusal adjustment after dental implant placement. Unfortunately, at the time of this study, little was known about the relationship between mandibular distortion and intermaxillary disproportion.
With these things in mind, we completed this study to determine stress distribution and distortion patterns during lateral movements of the mandible in Class I, II, and III intermaxillary relationships. In the following, we describe our particular method for FE analysis combining cone-beam computed tomographic (CBCT) imaging, 3-D scans of casts of subjects' dental impressions, and motion capture. We then discuss our results and the context into which our results and methodology fit, as well as implications and future directions for this work.
MATERIALS AND METHODS
All authors were well informed of the WMA Declaration of Helsinki - Ethical Principles for Medical Research Involving Human Subjects and confirm that the present study firmly complied with the declaration. This study was reviewed and approved by the Ethics Committee of Yonsei University (IRB no. 13-0070). Five Korean volunteers with no dental prostheses or facial deformities were selected as subjects. The subjects were classified by intermaxillary position (Class I, II, or III) according to the relationship between the mesiobuccal cusp (MBC) of the mandibular 1st molar and the interdental space between the MBC of the maxillary 1st molar and the 2nd premolar. Case numbers were assigned as follows:
Case I: subject with normal Class I occlusion.
Case II: subject with Class II occlusion (retrusion of mandible) and open bite.
Case III: subject with Class II occlusion (retrusion of mandible) and deep bite.
Case IV and V: subjects with Class III occlusion (protrusion of mandible).
The process for imaging the subjects’ skulls followed the protocol described in our previous research by Park et al. [
5]. The skulls of the subjects were scanned using CBCT equipment (Ray Co., Hwaseong, Korea) with a scanning increment of 2 mm (a total of 20 image slices were taken) and the images were processed using MIMICs software (Materialise Co., Leuven, Belgium). Uploaded images of the skulls and surrounding muscles were separated by the software, and only skull images were used for analysis. Extracted skull information was analyzed with Solidworks software (Dassault Systemes Solidworks Co., France). Individual cast models were created from dental impressions of the subjects and scanned with a 3-D optical laser scanner KOD-500 (Orapix, Seoul, Korea). The dentition images were processed with MeshLab software (Visual Computing Lab, Pisa, Italy) and uploaded into MIMICs. Files of dental images were transferred to Blender 3-D software (Blender, Amsterdam, Netherlands). Five complete images of the skull and teeth for each subject were selected as rigid components for FE analysis.
Mandible motion simulation was based on our previous research [
5]. The trajectory of the incisor on the lateral side (anteroposterior aspect) and on the anterior side (mediolateral aspect) during right side excursion movement was investigated using mandibular motion images obtained with the optical motion capture system (Visol Inc., USA) and the motion analysis system (Kwon 3D, Seoul, Korea) (
Figure 1). The stress distribution in and distortion of the mandibles were examined at the time (time of closing contact; TCC) when the teeth on both sides were in contact during the closing phase of unilateral mastication. The occlusion contact between the maxilla and mandible was also recorded at the TCC (
Figure 2). Weights of 100, 150, 200, and 250 kg (908 N, 1,470 N, 1,960 N, and 2,205 N) were applied. The direction of load application during lateral movement was aligned according to incisor motion information for each subject. Stress distribution and distortion were analyzed by means of ANASYS software (ANASYS Inc., USA) (
Figure 3 and
4). The material specifications of the bone were designated as in our previous study (Young’s modulus at 2.2059e+011 N/m
2, shear modulus at 9.0407e+010 N/m
2 tensile strength at 1.7234e+008 N/m
2, compressive strength at 5.5149e+008 N/m
2) [
5].
Figure 1
The trajectory of an incisor during lateral movement. (A) The motion of an incisor was captured from the anterior side. (B) The motion of an incisor was captured from the lateral side.

Figure 2
The reproduction of occlusion contacts (red colored area) at the time of initial contact on both sides during the closing phase of lateral movement. (A) The 3D simulation of lateral movement was performed based on CBCT images and captured motion information. (B) Occlusal contacts were examined on the occlusal aspects of the reconstructed image.
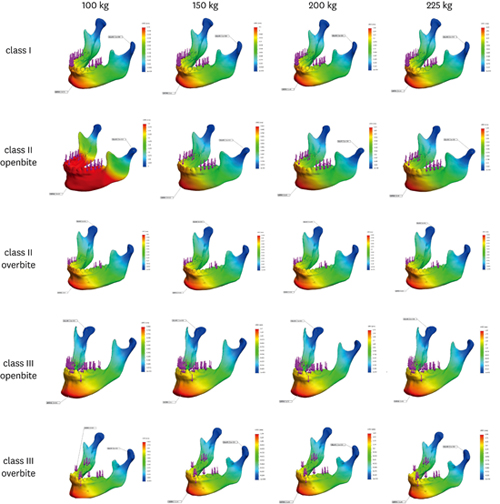
Figure 3
Stress distribution with simulation of lateral movement with 100, 150, 200 and 225kgf loading force in cases I~V.

Figure 4
Distortion of mandible with simulation of lateral movement with 100, 150, 200 and 225kgf loading force in cases I~V.

RESULTS
Points of occlusion contact at the TCC during lateral movement differed among the cases (
Table 1). In case I, even contact from the central incisor to the 2
nd molar was observed on the working side, whereas only the central incisor and molars made contact on the balancing side. Occlusal contacts were shown to be more localized in other cases (Classes II and III). In the Class II relationship (cases II and III), occlusal contacts on the working side were anterior to those on the balancing side, similar to the Class I occlusion. In the Class III relationship (cases IV and V), occlusal contacts on the balancing side were broader than on the working side.
Table 1
Occlusal contacts at the closing phase of lateral movement

|
Cases |
Working side (Right) |
Balancing side (Left) |
|
M2 |
M1 |
PM2 |
PM1 |
C |
LI |
CI |
CI |
LI |
C |
PM1 |
PM2 |
M1 |
M2 |
|
Class I |
Case I (normal occlusion) |
|
o |
o |
o |
o |
o |
o |
o |
|
|
|
|
o |
o |
|
Class II |
Case II (open bite) |
|
o |
o |
|
o |
|
|
|
|
|
|
|
o |
o |
|
Case III (deep bite) |
|
|
o |
o |
o |
o |
|
|
|
|
|
|
o |
o |
|
Class III |
Case IV |
o |
o |
o |
|
|
|
o |
o |
|
o |
|
o |
o |
o |
|
Case V |
|
o |
|
o |
|
|
|
|
|
|
o |
o |
|
o |
The maximum value of the stress distribution (Von Mises’ stress) during lateral movement is shown in
Table 2. The maximum stress increased with loading force in all cases (
Figure 3). The location of maximum stress loading in Class I and II (cases I, II and III) was the condyle of the balancing side (
Figure 3A and B). In contrast, the maximum stress load occurred on the condyle of the working side in Class III (cases IV and V,
Figure 3C).
Table 2
The maximum value of the stress distribution (Von Mises’ stress) during lateral movement and its loading area

|
Objects |
Location of maximum stress loading area |
Values (x E+08 N/m2) |
|
100 kgf |
150 kgf |
200 kgf |
225 kgf |
|
Class I |
Case I |
Condyle (superior side) on the balancing side |
4.09 |
6.14 |
8.18 |
9.20 |
|
Class II |
Case II
(open bite) |
Condyle (anterior side) on the balancing side |
11.40 |
17.10 |
22.80 |
25.60 |
Case III
(deep bite) |
4.17 |
6.26 |
8.34 |
9.38 |
|
Class III |
Case IV |
Condyle (anterior side) on the working side |
7.01 |
10.50 |
14.10 |
15.80 |
|
Case V |
4.13 |
6.19 |
8.25 |
9.29 |
Maximum distortion of the mandible during lateral movement is shown in
Table 3. Maximum distortion was observed on the menton at the midline, regardless of loading force, in all cases (
Figure 4). Distortion was greatest in Class III and least in Class II when observed within the same loading force group (
Figure 5).
Table 3
The maximum distortion of the mandible during lateral movement

|
Objects |
Values (mm) |
|
100 kgf |
150 kgf |
200 kgf |
225 kgf |
|
Class I |
Case I |
0.73 |
1.10 |
1.46 |
1.64 |
|
Class II |
Case II
(open bite) |
0.62 |
0.92 |
1.23 |
1.38 |
Case III
(deep bite) |
0.43 |
0.64 |
0.86 |
0.96 |
|
Class III |
Case IV |
0.87 |
1.30 |
1.73 |
1.95 |
|
Case V |
0.83 |
1.24 |
1.66 |
1.86 |
Figure 5
The maximum distortion of mandible according to the loading force in each case.

DISCUSSION
The gonial angle, ratio of mandibular horizontal length and dentition, and tooth contact distribution differ significantly according to the anteroposterior position of the mandible. All of these parameters have been shown to be meaningful factors affecting stress distribution in the mandible. For instance, Throckmorton et al. [
12] suggested that a larger gonial angle for the Class III position would result in a smaller perpendicular force to the occlusal plane and that the elevator muscles were inserted at a lower level on the mandible compared to the positions in Classes I and II.
In the present study, stress distribution patterns provided a valid means for locating the points of maximum loading and the sizes of the loading force for each subject. Distortion of the subjects’ mandibles was greater on their anterior portions than on their posterior portions with maximum distortion observed near the menton in all cases. Although occlusal force was greater on posterior teeth than on anterior teeth, due to the class III principle of leverage, torque was greater at the anterior portion of the mandible than at the posterior portion [
13].
In case I (normal occlusion), all dentition except for the 2nd molar on the working side were in contact at the TCC, while only molars on the balancing side were in contact. Stress on the working side was evenly distributed. The condyle on the balancing side absorbed more stress than on the working side. Although maximum stress increased with loading force in this case, it was loaded on the condyle of the balancing side regardless of the loading force applied.
In cases II and III (Class II occlusion with open bite and deep bite, respectively), observed tooth contact was more localized for the posterior dentition, and maximum stress was observed on the condylar neck of the balancing side. In cases IV and V (Class III occlusion), maximum stress was observed on the condylar neck of the working side. In Class III cases, the dentition was further from the axis (condylar head on the working side) than in Classes I and II. Thus, the stress distribution effect of tooth contact was less. Mandible distortion was greatest in Class III and smallest in Class II. We hypothesize that the ratio of mandibular length and dentition and the height of muscular attachment are related to the difference in distortion.
Unilateral mastication with lateral movement of the mandible is properly achieved by a rhythmic asynchronous contraction of masticatory muscles [
9]. The pterygoid muscles, in particular, play a key role in unilateral mastication [
1415]. Since the lateral and medial pterygoid muscles (LPt and MPt) lie deep inside the mandibular ramus, the pathway of an EMG probe is likely to be disturbed by the ramus or affected by the masseter muscle. Furthermore, the position of the mandible affects the EMG activity of the pterygoid muscles [
16]. Thus it is very difficult to conduct EMG recording studies on this muscle. The inferior head of the lateral pterygoid muscle has a reciprocal role with the medial pterygoid muscle during chewing and contributes to forward and lateral bracing of the condyle of the mandible. Although the superior head of the lateral pterygoid muscle appears to be active during mandibular closing, the significance of this finding is not fully understood [
17]. The two heads of the LPt can also be divided into distinct quadrants according to the site of attachment and innervation [
8]. Due to the close spatial relationship between the LPt and the TMJ, the activation of different LPt segments plays a role in modulation of TMJ function. Therefore one can speculate that hypertension of the Lpt can occur with bruxism or aberrant occlusion.
Yamaguchi et al. [
10] performed a muscle fMRI study and reported that the ipsilateral masseter, ipsilateral medial pterygoid, contralateral LPt, and contralateral masseter muscles were significantly activated during unilateral movement, with the muscles listed in order of activity. The masseter and medial pterygoid contract strongly on the working side, while the LPt does the same on the balancing side. In the present study, maximum stress was loaded on the anterior side of the condylar neck where the LPt muscle was inserted. Thus our results correspond with the previously established claim that the LPt muscle contributes to stabilizing the TMJ during lateral movement. It is interesting that, in Class III cases, maximum stress occurred on the working side, unlike in Class I and Class II cases. This may imply that the function of the LPt differs according to the anteroposterior position of the mandible. More detailed studies will be required to evaluate this hypothesis.
Motion and distortion of the mandible reflect various anatomical factors. These include not only the hard tissue frame, the craniofacial skeleton, the teeth and the like, but also soft tissue elements such as masticatory muscles and the TMJ disc. Muscular elements apply force to the craniofacial skeleton in various directions and the rendered net force moves the mandible along a path that the hard tissue frame allows. Direct measurement of occlusal force using a pressure-sensing film provides a remarkable means to determine a patient’s occlusion [
13]. Simulation studies, like the FE analysis performed here, however, complement such direct techniques. They provide a way to evaluate the individual effects of morphological factors on bone distortion and force distribution, excluding the mental effect on occlusal force.
The most important factor in FE modeling is reproducing the route of mandibular motion with reasonable material properties and morphology. The present study obtained a detailed morphology of each subject’s mandible and the attached teeth by means of CBCT and 3-D scanned dental images. Mandible morphological information gleaned from CBCT imagery is appropriate for reproduction of mandibular motion. Artifacts and low resolution, however, make it difficult to obtain detailed information on dentition with CBCT. Instead, 3-D scans of casts made from each subject’s dental impressions were used to reproduce their dental occlusions.
In this study, the anteroposterior and mediolateral aspects of the path traveled by teeth during chewing were obtained using a motion capture system. The data was used to reproduce mandibular motion. The trajectory of the incisor on the coronal plane during lateral movement in our study traced out a tear-drop shape (
Figure 1). Previous studies have suggested that such a path is normal [
1416]. Previous studies using FE analysis with 3-D modeling implicated muscle dimension and position as factors determining force magnitude and direction in the motion of the mandible. Commisso et al. [
9] performed FE modeling of the mastication cycle for awake and sleep bruxism, with the mandibular model created from a human cadaver and the orientation and magnitude of the muscles taken from other research data. Although the comparison of two simulations with different muscle activation patterns can provide useful clinical insight into activity harmful to the TMJ, a kinetic model based upon different sources of mandibular and muscular morphology is inappropriate for reproducing exact occlusal force.
Even recently, it was difficult to obtain exact 3-D representations of muscles. But now, researchers have digitized target muscles to investigate muscle dimension and position. Agur et al. [
18] produced a 3-D model of the soleus muscle from cadavers by digitizing muscle fibers. Lemos et al. [
19] digitized a cat’s medial gastrocnemius muscle for use in FE modeling and investigated architectural changes during muscle contraction. The intricate activities of masticatory muscles and the viscoelastic behavior of the TMJ, however, make it difficult to simulate the masticatory cycle using only dimensional information [
1011].
Taking a different approach, some previous studies used parameters obtained from the actual motion of living subjects as kinetic factors. In 2009, Röhrle et al. [
20] employed a motion capture system to record dynamic articulation. In their study, markers were attached to 3 points on the subject’s lower face and the paths of the markers were recorded. Likewise, Park et al. [
5] performed a simulation of masticatory movement using 3-D models constructed from a computed tomographic (CT) image of a volunteer’s mandible combined with mandibular motion data collected using an optical motion capture system. In our study, the trajectory of the incisor path of a living subject was selected as a parameter for mandibular motion. Given that it was based on a net motion rendered by various complicated muscular vectors, the motion model created here could prove more reliable in reproducing mastication than previous models developed using theoretical muscle vectors.
The present study has several limitations. TMJ viscoelasticity, the effect of periodontal space on the absorption of stress, and the root morphology of teeth likely affect distortion of a mandible. Larger samples should be investigated to establish a more reliable theory for mandibular distortion during unilateral mastication. Despite these limitations, the present study introduces a useful method combining FE analysis with a motion capture technique, and the results suggest that stress distribution and the accompanying distortion of the mandible might be affected by the anteroposterior position of the mandible.
In conclusion, FE analysis of an individual occlusion, performed alongside motion capture, may become a useful tool for gathering kinetic information related to an implant fixture and its involved structure, including opposite teeth and the jaw bone. In particular, the technique may inform dental implant treatment by describing the important distributions of stress and strain on the implant and its involved structures.









 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download