INTRODUCTION
Periodontitis is an inflammatory disease mainly caused by the growth of bacteria that attach to the surface of teeth [
1]. Recently, various protein biomarkers in human saliva have been studied for the prevention and treatment of periodontal diseases [
2]. Human saliva is a pivotal part of the immune defense system and contains roughly between 2,000 and 3,000 distinct salivary proteins, including alpha-amylase, lysozyme, mucins, proline-rich proteins, immunoglobulins, and many others [
34]. Among the wide range of salivary proteins, human common salivary protein 1 (CSP1, synonyms: ZG16B, HRPE773, PRO1567) has been identified as a homolog of the demilune cell and parotid protein (Dcpp) in mice and of CSP1 in rats [
56]. Kim et al. [
7] recently reported that the pancreatic adenocarcinoma up-regulating factor gene has a high degree of homology to CSP1 and that this novel secretory protein is involved in the progression of pancreatic cancer, suggesting a strong connection between systemic disease and oral disease. In fact, some studies have provided support for the proposal that diabetes functions as a risk factor for periodontal diseases [
8910].
So far, to the best of our knowledge, only a single article regarding the function of CSP1 in the field of dental research has been published, demonstrating that CSP1 may play a role in promoting the binding of
Streptococcus mutans (
S. mutans) to experimental salivary pellicles formed on a hydroxyapatite surface, influencing the initial colonization of this pathogenic bacterium [
1112]. However, further functional information on CSP1 regarding periodontal pathogens remains to be elucidated. In this study, a house-fabricated sandwich enzyme-linked immunosorbent assay (ELISA) system was used as a key method for measuring CSP1 levels in human saliva. Using this method, the concentration of salivary CSP1 was compared between healthy subjects and periodontal patients. The findings of this study lead to the preliminary proposal of salivary CSP1 as a potential biomarker for the screening or monitoring of periodontal diseases.
MATERIALS AND METHODS
Study design and clinical measurements
A controlled clinical study was conducted among healthy subjects and periodontitis patients with Institutional Review Board approval (No. 201512012) at Chonbuk National University Hospital from March 2013 to March 2014. All participants between 20 and 70 years of age were assessed for eligibility for our study after a basic medical and dental examination. Participants were excluded if they had any known systemic diseases influencing their periodontal status, such as diabetes or cardiovascular disease. Although smoking history was recorded, smokers were not excluded. All clinical parameters, including bleeding on probing, pocket depth, and clinical attachment level, were measured by a periodontist. Periodontal diagnoses were made using the criteria defined at the international workshop for a classification of periodontal diseases and conditions [
13]. An analysis of the previous literature showed that 16 samples each for the controls and cases were enough to ensure statistical significance for CSP1 measurements [
14]. Therefore, 36 systemically and periodontally healthy controls and 33 periodontal disease patients signed a written informed consent form and were enrolled into the present cross-sectional study (
Figure 1). The clinical characteristics of the study groups are presented in
Table 1.
Figure 1
Flow diagram of the study. This controlled study was conducted in periodontally healthy subjects and in patients with chronic periodontitis. All participants between 20 and 70 years of age were asked to take part in the study, agreed, and signed a written consent form. After providing informed consent, patients received a periodontal examination. The periodontal examinations were used to determine whether each patient belonged to the healthy group or to the group of patients with periodontitis.
CSP1, common salivary protein 1; ELISA, enzyme-linked immunosorbent assay; n, number.
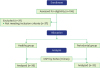
Table 1
Demographic characteristics of the study groups

|
Characteristics |
Healthy group |
Periodontal group |
P value |
|
Total |
36 (52.2) |
33 (47.8) |
|
|
Gender |
|
|
>0.100 |
|
Male |
21 (58.3) |
23 (69.7) |
|
Female |
15 (41.7) |
10 (30.3) |
|
Smoking |
|
|
0.540 |
|
Smokers |
8 (22.2) |
19 (57.6) |
|
Nonsmokers |
28 (77.8) |
14 (42.4) |
|
Severity of periodontitis |
|
|
0.024 |
|
Healthy |
36 (100) |
0 |
|
Mild |
0 |
10 (30.3) |
|
Moderate to severe |
0 |
23 (69.7) |
|
Age (yr) |
34.30±9.70 |
47.00±12.00 |
|
|
PD (mm) |
0.70±0.06 |
4.46±1.72 |
<0.001 |
|
CAL (mm) |
1.40±0.16 |
4.25±2.51 |
<0.001 |
|
BOP (%) |
31.76 |
66.67 |
<0.001 |
Saliva collection and storage
All participants were advised not to eat food or drink any beverages for at least 1 hour before sample collection. Saliva samples were collected in 50 mL polypropylene conical tubes and frozen at −70℃. When all the saliva samples were obtained, they were thawed at room temperature, divided into 1.5 mL Eppendorf tubes, and centrifuged at 13,000 g at 4℃ for 15 minutes [
15]. The supernatant was saved and transferred to new 1.5 mL tubes, and the samples were frozen at −70℃ until they were ready to be used.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting
Proteins were denatured by boiling for 1.5 minutes after adding 2× sodium dodecyl sulfate (SDS) treatment buffer and then were separated on a 12% SDS-polyacrylamide gel. Proteins on the gels were either stained with Coomassie blue or electro-transferred to a polyvinylidene membrane for immunoblotting [
15]. The blot was blocked with 5% nonfat powdered milk in phosphate-buffered saline buffer with 0.2% Tween 20 (PBST) and probed with biotin-conjugated monoclonal antibody (mAb) to CSP1 (mAb-hCSP1#4) for 2 hours at room temperature [
13]. After the membrane was thoroughly rinsed with PBST buffer, the blot was visualized using streptavidin conjugated to horseradish peroxidase (HRP) and an enhanced chemiluminescence substrate kit (Pierce, Rockford, IL, USA). CSP1 protein was detected by exposure of the blot to Biomax-MS X-ray film (Eastman Kodak, Rochester, NY, USA) in a dark room.
Standard curve for ELISA
A standard curve for CSP1 levels in human saliva using a sandwich ELISA system was obtained with different concentrations of recombinant hCSP1 (0–14,000 ng/mL). In the standard curve, the optical density value at 450 nm was plotted on the y-axis against the hCSP1 concentration on the x-axis. In order to detect hCSP1 as a standard protein, mAb-hCSP1#14 and mAb-hCSP1#4 were used as the capture mAb and the detector mAb, respectively. A reliable Pearson correlation coefficient value (r
2=0.983) was observed between the 2 parameters, indicating good linearity throughout the entire measurement range (data not shown).
Sandwich ELISA
Sandwich ELISA was used in this study to measure the concentration of CSP1 in saliva. In brief, 50 µL of capture mAb (mAb-hCSP1#14, 2 µg/mL) was applied to the ELISA plate, and the plate was incubated for 1 hour at 37℃ and washed 3 times with PBST. After the plate was treated with 50 µL of bovine serum albumin blocking solution (10 mg/mL), 50 µL of the serum was added to the wells, and the plate was incubated for 1 hour at 37℃. After washing the plates with PBST, 50 µL of the biotin-conjugated detector mAb (mAb-hCSP1#4, 2 µg/mL) was added to the wells and incubated for 1 hour at 37℃ prior to being probed with an avidin-HRP solution (10 mg/mL). Following a final rinse with PBST, the color reaction for the detection of antigen-mAb complex was initiated with the addition of 50 µL of 3,3',5,5'-tetramethylbenzidine substrate solution (10 mg/mL) for 15 minutes and then stopped by adding 50 µL of 10 µmol/mL H2SO4. The absorbance was measured at 450 nm in an automatic ELISA reader (Model 550, Bio-Rad, Hercules, CA, USA).
Data analysis
To determine the normality of the distribution of the sample, the D’Agostino and Pearson omnibus normality test was performed using PRISM version 6 (GraphPad Software Inc., La Jolla, CA, USA). PASW Statistics for Windows version 18.0 (SPSS Inc., Chicago, IL, USA) and Microsoft Excel 2010 (Microsoft Corp., Redmond, WA, USA) were used to analyze and compare the test results. P-values <0.05 were considered to indicate statistical significance. Pearson correlation coefficients (r
2) were used to evaluate correlations between 2 parameters.
DISCUSSION
Saliva is thought to act as an indicator of oral disease, including periodontitis. However, our knowledges of whether and which salivary proteins are affected by the processes of periodontal disease remain limited. In this study, the subjects were divided into 2 groups, periodontally healthy individuals and periodontitis patients, after a comprehensive periodontal examination (
Figure 1,
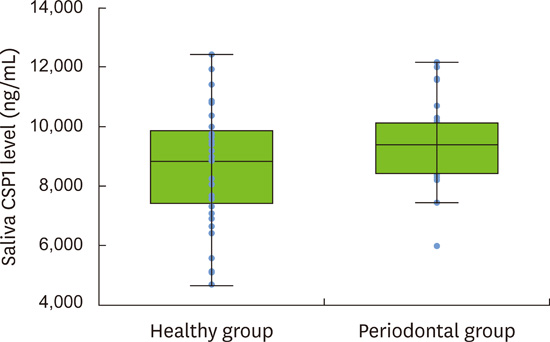
Table 1). Then, salivary levels of human CSP1, a major salivary protein, were measured in both healthy individuals and periodontitis patients. CSP1 levels were measured using a custom-fabricated sandwich ELISA system, and were compared and analyzed (
Table 2,
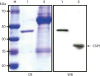
Figure 3). As a capture antibody and a detector antibody, mAb-hCSP1#14 and mAb-hCSP1#4 were tested for their binding capacity by immunoblotting with human saliva (
Figure 2) and evaluated using the Pearson correlation coefficient with the standard curve.
SDS-PAGE and western blot analysis showed that the molecular weight of recombinant hCSP1 tagged with GST was approximately 46-kDa, as indicated in lane 1 of
Figure 2. Since the molecular weight of GST is known to be 26-kDa, the molecular weight of CSP1 was estimated to be approximately 20-kDa. However, mAb-hCSP1#4 recognized only a single band at approximately 27-kDa in human saliva, as shown in lane 2 of the western blot in
Figure 2, which is somewhat larger than was predicted from the CSP1 gene sequence. This result is consistent with previously reported data [
1115]. Ambatipudi et al. [
11] explained this discrepancy by noting that the human CSP1 sequence contains one potential N-glycosylation site, and thus human CSP1 may exist in a glycosylated form in saliva. Dcpp in mice, a homolog of human CSP1, has 3 forms encoded by different genes [
5], but rat CSP1 is encoded by only 1 gene, as in humans. In the case of rats, interestingly, 3 CSP1 isoforms with molecular weights of 16-kDa, 20-kDa, and 22-kDa, were identified in the salivary glands, suggesting that different isoforms of CSP1 may have been derived from N-glycosylation or phosphorylation of the CSP1 protein [
6].
In the present study, the
t-test was used to examine whether the mean differences between the 2 groups were statistically significant because the values of CSP1 concentrations exhibited a normal distribution. The means (standard deviations) of saliva CSP1 concentrations in 36 healthy subjects and 33 periodontal patients were 8,598 ng/mL (1,932 ng/mL) and 9,474 ng/mL (1,290 ng/mL), respectively (
Table 2,
Figure 3), showing that the difference in CSP1 levels between the 2 groups was statistically significant (
P=0.024). No statistically significant intragroup differences in CSP1 levels were found, for example, smoking vs. non-smoking (
P=0.400) or mild vs. moderate to severe periodontitis (
P=0.800) in periodontal patients or smoking vs. non-smoking (
P=0.200) in healthy subjects. At this point, the reason why CSP1 levels were higher in periodontal patients than in normal healthy subjects remains unknown.
One possible explanation for the presence of a higher level of CSP1 in periodontal patients than in healthy subjects can be deduced from the presence of a putative jacalin-related lectin (JRL) domain in human CSP1. Members of the JRL protein family are known to bind to glycoproteins, are ubiquitously expressed throughout the plant and animal kingdoms, and carry out functions such as cell agglutination and antimicrobial activity [
516]. Thus, CSP1 may play a role in modulating the composition of oral microflora, similarly to other salivary proteins such as lysozyme and lactoperoxidase, by binding pathogenic bacteria [
17] and causing aggregation and direct killing. Although rat CSP1 includes a JRL domain, it did not show any detectable effects on cell agglutination and antimicrobial activity, but rather led to enhanced bacterial adherence when
S. mutans were exposed to rat CSP1 in saliva [
12]. Human CSP1, similarly to amylase, may encourage pathogenic bacteria to bind to the tooth surface and act as bacterial anchor sites of initial pathogen tooth colonizers [
18], suggesting that the presence of other salivary proteins may mediate the attachment of CSP1 to bacterial surfaces or between the bacteria and salivary pellicles, possibly by forming nanostructure protein complexes. Thus, another explanation for the higher level of CSP1 in periodontal patients than in healthy subjects may be related to the higher stress level associated with chronic diseases. As several recent studies have shown that that CSP1 was highly expressed in stressed conditions, including periodontitis, pancreatic cancer [
7], and diabetes mellitus [
14], chronic stress may act as a risk factor for higher CSP1 levels in chronic periodontitis. Whether chronic stress directly affects the level of CSP1 remains an intriguing question to be resolved.
Most importantly, the present study showed that periodontitis patients had a significantly higher level of salivary CSP1 than healthy subjects. Our findings may suggest that CSP1 could be used as a potential biomarker for the detection or screening of periodontal patients. Due to the advantages of saliva, including non-invasiveness, safety, and simplicity, early diagnosis and treatment of periodontal disease may become more accessible [
19]. As a future strategy, a much larger randomized controlled cohort study [
20] is required.
In conclusion, human CSP1 was identified in saliva as a single 27-kDa band by probing with mAb-hCSP1#4 and immunoblotting. The mean (standard deviation) CSP1 levels in healthy controls and periodontal patients were 8,598 ng/mL (1,932 ng/mL) and 9,474 ng/mL (1,290 ng/mL), respectively, and a statistically significant difference in CSP1 levels between the 2 groups (P=0.024) was observed using a sandwich ELISA system. These results indicate that CSP1 may be used in the future as a potential biomarker for the detection and screening of periodontal patients.







 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download