Abstract
Purpose
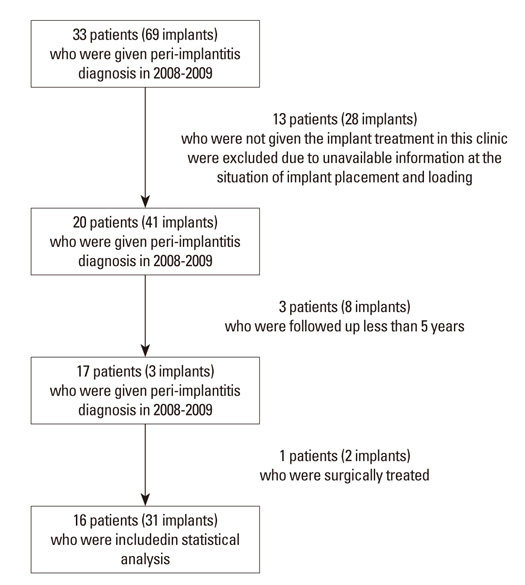
Methods
Results
Figures and Tables
Figure 2
Measurements of the marginal bone level. a: Marginal bone level on the mesiobuccal side of implant (mm), b: Marginal bone level on the mesiolingual side of implant (mm), c: Marginal bone level on the distobuccal side of implant (mm), d: Marginal bone level on the distolingual side of implant (mm).
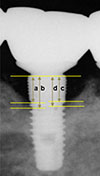
Figure 3
Outline of this study. Radiographic measurements were performed at each interval (A, B, and C). Values are representing mean±SD months at each interval.

Figure 4
Radiographs of a 57-year-old woman who had an implant diagnosed as peri-implantitis. a) radiograph at implant loading, b) radiograph at peri-implantitis diagnosis, c) radiograph 61 months after peri-implantitis treatment.

Table 1
Characteristics of patients included in this study.

Table 2
Characteristics of implants included in this study.

a)Periods until diagnosis of peri-implantitis was defined as periods from 2 weeks recall-check for re-tightening after final prosthesis delivery to diagnosis of peri-implantitis.
b)The percentage of simultaneous lateral sinus lift was calculated among implants placed in maxillary premolar and molar regions (n=12).
Table 3
Radiographic marginal bone level at each interval of non-surgically treated implants with more than 5 years follow-up periods after peri-implantitis diagnosis.

Paired-t test was conducted to compare bone levels at each interval (P-values are corrected by Bonferroni's method due to multiple testing, significant level was P<0.05/3).
a)Initial bone level was defined as bone level at 2 weeks recall-check for re-tightening after final prosthesis delivery.
b)A statistical difference from (B) and (C) [(A) and (B) P<0.001; (B) and (C) P<0.001].
c)A significant difference between (A) and (B) [(A) and (B) P<0.001) ; (A) and (C) P=0.016].
d)A statistical difference from (C-B) and (C-A) [(B-A) and (C-B) P=0.002; (B-A) and (C-A) P<0.001].
e)A significant difference between (B-A) and (C-B) [(C-B) and (B-A) P=0.007; (C-B) and (B-A) P=0.007].
Table 4
Marginal bone levels at each interval according to the factors.

Marginal bone levels at each interval were normally distributed and parametric analysis was performed. To compare independent groups, Student t-test or ANOVA with Tuckey's post hoc test was done. Values were measured by mm unit.
CVD, cardiovascular disease; DM, diabetic mellitus.
a)Statistically significant difference (P<0.05).
Table 5
Mixed effect regression analysis to investigate the marginal bone loss of implants (n=31).

a)Statistically significant difference (P<0.05).
Mixed model regression analysis was performed to investigate the effects of variables on the amounts of bone loss (fixed variables - timing of peri-implantitis diagnosis, bone level at loading, gender, systemic disease, periodontal status, location of implants, surgical factors, random variables - patients). Regression model for initial bone loss was P<0.001, P<0.001 for additional bone loss and P<0.001 for total bone loss.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download