INTRODUCTION
In a cohort study by Roos-Jansaker et al. [4] 14%-16% of the patients had at least one implant with radiographic bone loss of at least 3 threads and a pocket depth of ≥6 mm after being followed for 9-14 years.
With the threshold of bone loss defined as the presence of at least three threads in at least one implant, Fransson et al. [5] reported that 28% of their patients showed radiographic bone loss over 5-20 years. Further, out of the 3,413 implants included in the study, 12.4% demonstrated progressive bone loss.
Ferreira et al. [6] defined peri-implantitis as the presence of a PPD >4 mm in association with peri-implant bleeding and/or suppuration with radiographic confirmation of bone loss; 9% of their patients were diagnosed with peri-implantitis.
Koldsland et al. [7] found a wide variation in peri-implant prevalence, 11%-47%, dependant on the radiographic interpretation of peri-implant bone loss and PPD threshold limit. With a PPD ≥4 mm, BOP, and radiographic bone loss ≥2 mm, 20.4% of their patients had peri-implantitis. When the threshold was increased to a PPD ≥6 mm with BOP and radiographic bone loss ≥3 mm, the prevalence of peri-implantitis decreased to 11.3%.
In a systematic review by Jung et al. [8] on the 5-year survival and complications of peri-implantitis, the incidence of the disease at single crowns was 9.7% . Further, bone loss >2 mm occurred in 6.3% of all implants over the 5-year observation period.
A recent systematic review [9] considering the biological and technical complications in implant dentistry revealed that the incidence of peri-implantitis increased with time; the 5- to 10-year incidence was greater than the 0- to 5-year incidence.
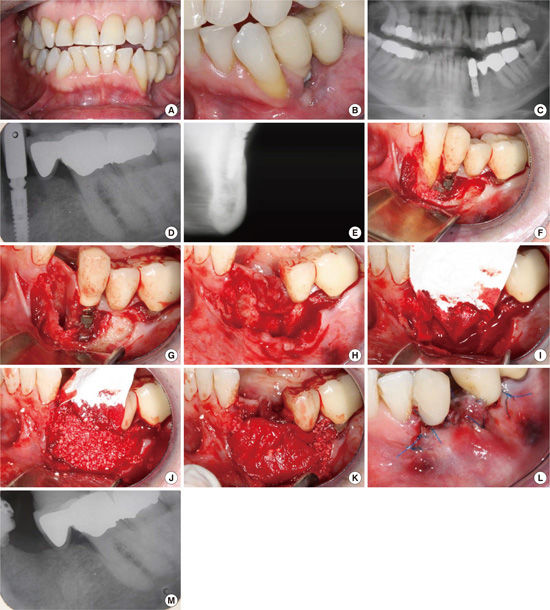
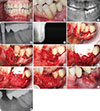
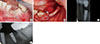
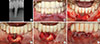
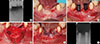
Early peri-implantitis, a PPD ≥4 mm with bleeding and/or suppuration on probing. Bone loss <25% of the implant length.
Moderate peri-implantitis, a PPD ≥6 mm with bleeding and/or suppuration on probing. Bone loss 25%-50% of the implant length.
Advanced peri-implantitis, a PPD ≥8 mm with bleeding and/or suppuration on probing. Bone loss >50% of the implant length.




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download