Abstract
Purpose
Methods
Results
Conclusions
Figures and Tables
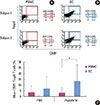 | Figure 1Fluorescence activated cell sorter profiles of peptide 14-specific regulatory T cells (Tregs) (CD4+, CD25+, and Foxp3+) in peripheral blood mononuclear cells (PBMCs) and dendritic cells (DCs) from two representative subjects (A, B) demonstrating the capability of DCs to enhance Tregs from 0.07% to 32.54% in subject 1 and from 0.15% to 34.46% in subject 2. The bar graph shows the mean percentage of peptide 14-specific Tregs of 10 subjects (C), evidencing a statistically significant difference between PMBCs and DCs (*P<0.05). Phosphate buffered saline (PBS) was used as a control antigen. UL: upper left panel, UR: upper right panel, LL: lower left panel, LR: lower right panel. |
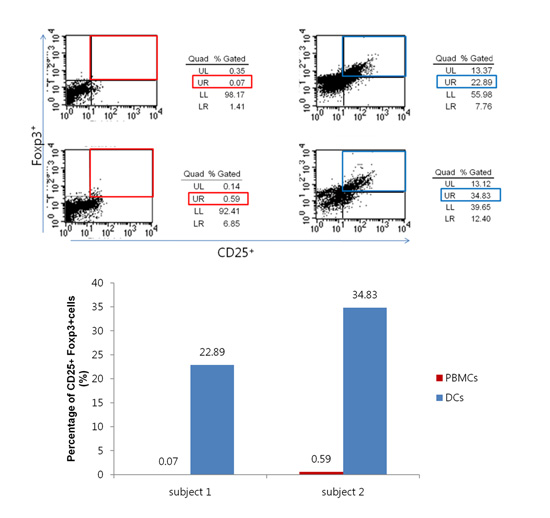
 | Figure 2Fluorescence activated cell sorter profiles of peptide 19-specific regulatory T cells (Tregs) (CD4+, CD25+, and Foxp3+) in peripheral blood mononuclear cells (PBMCs) and dendritic cells (DCs) from two representative subjects (A, B) demonstrating the capability of DCs to enhance Tregs from 0.07% to 22.89% in subject 1 and from 0.59% to 34.83% in subject 2, respectively. The bar graph shows the mean percentage of peptide 19-specific Tregs of 10 subjects (C). The difference between PMBCs and DCs was not statistically significant. Phosphate buffered saline (PBS) was used as a control antigen. UL: upper left panel, UR: upper right panel, LL: lower left panel, LR: lower right panel. |
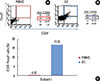 | Figure 3Stimulation of peptide-specific regulatory T cells (Tregs) by peripheral blood mononuclear cells (PBMCs) and dendritic cells (DCs). (A) Peptide number 19 from Streptococcus sanguinis heat shock protein 60 has shown a minimal capability (0.05%) to convert T cells into Tregs when PBMCs are used as antigen-presenting cells. (B) DCs were used for presenting peptide antigens, which enhanced induction of Tregs (17.02%). (C) The bar graph shows the percentage of peptide-specific Tregs among T cells in one subject. UL: upper left panel, UR: upper right panel, LL: lower left panel, LR: lower right panel. |
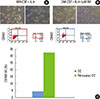 | Figure 4Comparison of effect of retinoic acid treatment with nontreatment on the phenotype expression of dendritic cells (DCs). (A) The morphology of DCs has demonstrated the typical extension of dendritic processes characteristic of maturing DCs when retinoic acid (RA) has been added to the culture medium. (B) The percentage of CD103+ cells was increased from 4.28% to 32.67%. (C) A bar graph summarizing the fluorescence activated cell sorter results is shown. GM-CSF: granulocyte-monocyte colony-stimulating factor, IL: interleukin, UL: upper left panel, UR: upper right panel, LL: lower left panel, LR: lower right panel. |
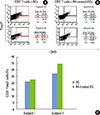 | Figure 5Comparison of effect of retinoic acid (RA) treatment with nontreatment on regulatory T cells (Tregs) induction by peptide number 19 from Porphyromonas gingivalis heat shock protein 60 (HSP60). (A) T cells were stained for CD4, CD25, and Foxp3 and analyzed by fluorescence activated cell sorter. The percentage of peptide-specific Tregs induced increased from 21.12% to 22.89% (subject 1 in C), and it increased from 27.73% to 34.46% (subject 2 in C) for peptide number 19 from P. gingivalis HSP60 by the addition of RA. (B) The bar graph shows the percentage of peptide-specific Tregs among T cells in two subjects. The difference between cases with and without RA failed to reach statistical significance. UL: upper left panel, UR: upper right panel, LL: lower left panel, LR: lower right panel. |
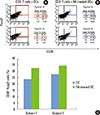 | Figure 6Comparison of the effect of retinoic acid (RA) treatment and nontreatment on regulatory T cells (Tregs) induction by peptide number 14 from Porphyromonas gingivalis heat shock protein 60 (HSP60). (A) T cells were stained for CD4, CD25, and Foxp3 and analyzed by fluorescence activated cell sorter. The percentage of peptide-specific Tregs induced increased from 23.70% to 32.54% (subject 1 in C), and it increased from 27.57% to 34.83% (subject 2 in C) for peptide number 14 from P. gingivalis HSP60 by the addition of RA. (B) The bar graph shows the percentage of peptide-specific Tregs among T cells in two subjects. The difference between cases with and without RA failed to reach statistical significance. UL: upper left panel, UR: upper right panel, LL: lower left panel, LR: lower right panel. |
Table 1





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download