Abstract
Purpose
Methods
Results
Figures and Tables
Figure 1
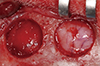
Figure 2

Figure 3

Figure 4

Table 1

| Treatment for the defect | Treatment for the adjacent defect | |
|---|---|---|
| CC | Blood clot only | BCP only |
| CB | Blood clot only | BCP + BMP-2 |
Journal List > J Periodontal Implant Sci > v.44(4) > 1082420





| Treatment for the defect | Treatment for the adjacent defect | |
|---|---|---|
| CC | Blood clot only | BCP only |
| CB | Blood clot only | BCP + BMP-2 |
Jin-Wook Lee 
https://orcid.org/http://orcid.org/0000-0002-0898-4599
Hyun-Chang Lim 
https://orcid.org/http://orcid.org/0000-0001-7695-1708
Eun-Ung Lee 
https://orcid.org/http://orcid.org/0000-0003-1900-870X
Jin-Young Park 
https://orcid.org/http://orcid.org/0000-0002-6408-1618
Jung-Seok Lee 
https://orcid.org/http://orcid.org/0000-0003-1276-5978
Dong-Woon Lee 
https://orcid.org/http://orcid.org/0000-0002-0796-9100
Ui-Won Jung 
https://orcid.org/http://orcid.org/0000-0001-6371-4172
Seong-Ho Choi 
https://orcid.org/http://orcid.org/0000-0001-6704-6124