INTRODUCTION
Chronic periodontitis is the most prevalent form of periodontitis, which affects a majority of the adult population and causes tooth loss [
1]. Tissue destruction that occurs during the disease process results in the release of various inflammatory mediators such as interleukin 1β, tumor necrosis factor-alpha, and prostaglandin E
2, which play a pivotal role in the loss of connective tissue as well as the supporting alveolar bone [
2]. These inflammatory mediators show variation in the concentration with the severity of periodontitis and may show a positive/negative correlation with disease progression [
3]. Hence, quantitative analyses of these components will be useful biomarkers for the diagnostic and prognostic assessment of periodontitis. Among the body fluids, gingival crevicular fluid (GCF) provides a unique window for the analysis of the periodontal condition. Because GCF is an inflammatory exudate that seeps into gingival crevices or periodontal pockets around the teeth, it crosses periodontal tissues and en route collects various inflammatory byproducts of potential interest from the local inflammatory reaction, which may be used as a marker for the diagnosis of periodontal disease activity. Apart from this, GCF collection is a noninvasive and relatively simple procedure to perform [
4].
Recently, the presence of a leptin molecule was reported in GCF [
5]. Leptin, a 16-kDa, nonglycosylated polypeptide produced primarily by adipocytes and released into systemic circulation, performs a multitude of regulatory functions involving energy use and storage, regulation of various endocrine axes, bone metabolism and thermoregulation [
6]. In addition to leptin's best-known role as a regulator of energy homeostasis, several studies indicate that leptin plays a pivotal role in immune and inflammatory responses [
7]. Leptin, an inflammation-sensitive protein, is associated with periodontal inflammation severity [
8]. Although adipocytes are absent in gingiva, a higher leptin concentration was found in healthy gingival tissue than in diseased gingiva [
9]. The GCF leptin concentration decreased substantially with increased periodontal destruction, suggesting a negative correlation of GCF leptin with clinical attachment loss [
9,
10]. However, in contrast to GCF leptin, serum leptin showed a positive correlation by an increase in its concentration as the severity of inflammatory periodontal destruction increased, and nonsurgical treatment was found to be effective in reducing its concentration [
11].
Advances in the understanding of the etiology and pathogenesis of periodontitis have led to increasingly effective pharmacological interventions either alone or along with surgical/nonsurgical mechanical therapy. Since systemic therapy has some disadvantages such as hypersensitivity reactions, organ toxicity, and development of resistant bacteria, a high dose requirement to achieve the necessary concentration at the target site has led to the invention of a local drug delivery (LDD) system. Among various LDD systems, tetracycline was found to be more effective due its broad spectrum of activity [
12], anticollagenolytic property [
13], capacity to delay pellicle and plaque formation [
14], ability to remove the root surface smear layer [
15], adherence capacity to tooth structure and active secretion at high concentrations in GCF [
15].
To evaluate the effects of therapeutic agents on localized defects, a split mouth study design is very useful as it reduces the influence of patient-specific characteristics and interpatient variability, thereby reducing the sample size for the study [
16]. The statistical efficacy of such a study increases when patients serve as their own control [
16].
Thus far, there have been no scientific reports regarding the effects of scaling and root planing (SRP) with and without LDD on the GCF leptin concentration of chronic periodontitis patients. Hence, the aim of this study was to estimate and compare the GCF leptin level before and after nonsurgical therapy (SRP) with and without LDD (tetracycline fibers; Periodontal Plus AB, Advanced Biotech Products, Chennai, India) in chronic periodontitis patients. The variations in the severity of inflammation were assessed with the clinical parameters and the estimation of the GCF leptin level using enzyme-linked immunosorbent assay (ELISA).
MATERIALS AND METHODS
For this proposed split mouth design study, a total of 90 sites from 30 nonobese adult chronic periodontitis patients within the age range of 30-60 years (mean age, 39.5 years) of either sex (13 males and 17 females) free from systemic diseases and with a body mass index (BMI) between 18.5 and 30 kg/m2, pocket depth of 4-6 mm, bleeding on probing, and radiographic evidence of bone loss on at least 2 teeth in a minimum of two quadrants were selected. Patients who were unable to perform the routine oral hygiene procedure, were unable to come for regular follow-up, had diseases of oral hard and soft tissues except caries and periodontal diseases, had undergone any periodontal or antimicrobial therapy in the past 3 months, were allergic to tetracycline drug, were pregnant and lactating mothers, and/or had a history of smoking, diabetes mellitus, alcohol consumption, malignancy, rheumatoid arthritis, and/or cardiovascular diseases were excluded from the study.
For each patient, a detailed verbal and written description of the study was given and a signed consent form to participate in the study was obtained. The study was approved by the Institutional Review Board of Bapuji Dental College and Hospital, Davangere, Karnataka, India, prior to commencement of the study.
The selected sites were divided into the following three groups:
Group I (control): consisted of 30 sites with clinically healthy periodontium and good oral hygiene status, (probing depth [PD], <3 mm; gingival index [GI] score, <25%) and did not receive any periodontal treatment.
Group II: consisted of 30 sites with periodontitis (pocket depth, 5-6 mm) and showing bleeding on probing, which were treated by SRP.
Group III: consisted of 30 sites with periodontitis (pocket depth, 5-6 mm) and showing bleeding on probing, which were treated by SRP, followed by tetracycline-impregnated collagen fiber LDD (Periodontal Plus AB).
Selected periodontitis sites were randomly allotted to groups II and III by using a lottery pick method. SRP is the initial phase of an orderly sequence of periodontal treatment; therefore, after scaling, residual embedded calculus and portions of necrotic cementum were removed in order to obtain a smooth, hard, and clean root surface by using appropriate Gracey curettes, and the thoroughness of root planing was confirmed by using an explorer.
Collection of GCF
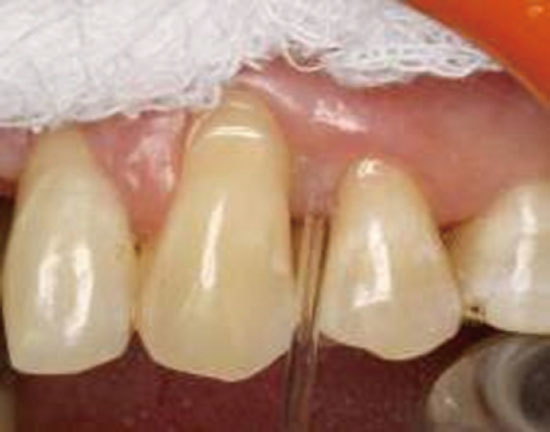
The selected test site was air-dried and isolated with sterile cotton rolls. Supragingival plaque was removed gently without touching the marginal gingiva to avoid bleeding from gingiva. By using an extracrevicular (unstimulated) method, a standard volume of 2 µL of GCF was collected from each selected site with a white color-coded, 1- to 5-µL calibrated volumetric microcapillary pipette (Sigma-Aldrich Chemie Gmbh, Munich, Germany) (
Fig. 1). The collected GCF samples were immediately transferred to the sterile Eppendorf tubes containing 98 µL of buffered alkaline phosphate saline and were immediately transported to the laboratory, where they were stored at -80℃ until the time of assay. Only the samples that were not visibly contaminated with blood or plaque were considered for the study.
Recording of clinical parameters
A blinded trained single periodontist (V.V.M) recorded all the clinical parameters and was calibrated for probing to a senior clinical researcher (D.S.M) before the study. Examiner calibration was considered effective for an interclass correlation coefficient of ≥0.9. For each patient, the plaque index (PI) [
17], gingival index (GI) [
18], and sulcus bleeding index (SBI) [
19] were recorded for all teeth, whereas the probing depth (PD), clinical attachment level (CAL), and gingival recession (GR) were considered only for the selected sites. At the selected tooth, probing was done at six sites. The sites that showed the deepest PD and the greatest CAL were chosen, and the same site was considered during follow-up visits. PD and CAL were measured with William's periodontal probe by using a customized acrylic stent. The lower border of the stent was used as the reference point for the CAL measurements (
Fig. 2).
Periodontal therapy
After the baseline (D0) GCF sampling and clinical parameter recording, all patients underwent thorough ultrasonic scaling and polishing. A trained clinician (A.B.D.) performed root planing with Gracey curettes (Hu-Friedy, Chicago, IL, USA) for both groups II and III sites. Saline-soaked tetracycline-impregnated collagen fibers (Periodontal Plus AB) were gently pushed to fill the periodontal pockets of group III sites (
Fig. 3), and the defect opening was sealed with periodontal dressing (Coe-Pac, GC America Inc., Alsip, IL, USA) to prevent ingress of oral fluids. Oral hygiene instructions (OHI) were given. Recall appointments were scheduled after 15 days (D15) and 45 days (D45) for follow-up. At each recall visit, GCF sampling and clinical parameter recordings were repeated by examiner V.V.M for group II and group III sites followed by reinforcement of OHI.
Leptin assay
The assay was performed by using BioSource Leptin-EASIA Kit (BioSource Europe S.A., Nivelles, Belgium) according to the manufacturer's instructions. It employed a quantitative sandwich enzyme immunoassay technique. The monoclonal antibody specific for leptin had been precoated onto the wells of the microtitre plate. Calibrator solution and GCF samples were pipetted into the wells, and the leptin present in the sample was bound to the immobilized antibody. After washing away the unbound substances, an enzyme-linked polyclonal antibody specific for leptin was added to the wells. Following a wash to remove the unbounded antibody-enzyme reagent, a chromogenic solution was added to the wells, which developed a color in proportion to the amount of leptin bound in the initial step. The color development was stopped by the addition of a stop solution, and the intensity of color was measured with the help of an ELISA color reader using 450 nm as the primary wavelength. The quantity of leptin in the samples was calculated by comparing with the standard calibrated curve included with the assay kit. The total leptin was determined in picograms (pg), and the concentration in each sample was calculated by dividing the amount of leptin by the volume of the sample (pg/mL).
Statistical analysis
SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA) was used to perform the statistical analysis. The mean values for the recorded clinical parameters were calculated. Simultaneous comparisons of PI, GI, and SBI were carried out by using the Friedman test, and Wilcoxon signed rank test was used for the pairwise comparison. The other clinical parameters were compared using the Student unpaired t-test, repeated measures analysis of variance, and Tukey test. A value of P<0.05 was considered statistically significant, and P<0.001 was considered statistically highly significant. Power analysis of the study was done using the Minitab software, and the resulting value was 83%.
DISCUSSION
Leptin, a 16 kDa nonglycosylated peptide hormone, has been classified as a cytokine as it shows structural similarities to the long-chain helical cytokine family [
6]. It has been suggested that leptin orchestrates the host response to infectious and inflammatory stimuli [
20]. It has emerged as a pleiotropic molecule involved in several physiologic and pathologic conditions [
21]. A potential link between obesity and periodontitis has been established by showing association between BMI and periodontitis [
22,
23]. Even a low-grade inflammation associated with obesity was found to obscure the clinical expression of local inflammation in the development of periodontitis [
8]. Obesity may act as a confounding factor; hence, for the present study, nonobese patients of BMI<30 kg/m
2 were selected for the study. Studies have reported an association between the severity of periodontitis and the leptin levels in serum or GCF [
5,
9,
10].
Johnson and Serio [
5] observed that leptin levels were highest in the healthy gingiva, which decreased with an increase in PD. This variation was attributed to the enhanced microvasculature found in periodontitis that caused the removal of leptin from gingival tissue and an increase in the serum leptin level. They suggested that the decrease in leptin levels in diseased gingiva was due to the increased microvasculature, which caused an increased rate of removal of leptin from the gingival tissue, thereby increasing the serum leptin level. They also suggested that a high gingival leptin concentration might be protective in nature for gingiva. Later, Bozkurt et al. [
24] evaluated the effects of long-term heavy smoking on GCF leptin levels in periodontitis patients and found that the GCF leptin level was significantly different when nonsmokers and smokers were compared with healthy individuals. The authors suggested the possible role of the leptin receptor expression in the gingiva during inflammation to explain the decrease in GCF leptin levels with increasing clinical inflammatory parameters.
Then, Karthikeyan and Pradeep [
9,
10] evaluated GCF levels in periodontal health and disease in patients with normal BMI. They concluded that GCF leptin levels decreased progressively from health to periodontitis and there was a strong negative correlation between the GCF leptin level and the progression of periodontal disease. Shimada et al. [
11] evaluated the effects of periodontal treatment on serum leptin, interleukin (IL) 6, and C-reactive protein (CRP). They found that at one-month follow-up, after nonsurgical therapy, serum leptin, IL-6, and CRP levels were significantly decreased. This revealed that there was a positive correlation between serum leptin levels and periodontal clinical parameters. In contrast, no association between the serum CRP level, IL-6, leptin, and adiponectin with periodontitis was observed even in morbidly obese patients [
8]. This is also supported by a case-control study in which no correlation between the decreased levels of serum adiponectin and periodontitis was found [
25].
To the best of our knowledge, the present study is the first one to use a split mouth study design to evaluate and compare the effects of two different nonsurgical therapies on GCF leptin levels along with various clinical periodontal parameters for a follow-up period of 45 days.
Mechanical and antimicrobial therapies, individually and in combination, have been shown to be effective at slowing or arresting the progression of periodontal disease [
26]. Antibiotics appear to be most useful adjuncts to mechanical therapy [
27]. Tetracycline, one of the most common antibiotics used in the treatment of periodontitis, has several features such as high concentration in the periodontal pocket, inhibition of collagenase [
13], and considerable substantivity [
28] on topical application, which contributes to its effectiveness against periodontal infection. Through a LDD system, safe and intrinsically efficacious medications can be delivered into periodontal pockets to suppress or eradicate the pathologic microorganisms [
13,
27] and modulate the inflammatory response [
13]. Local delivery of tetracycline has several advantages over systemic administration: (1) a relatively small amount of drug can produce high concentrations in the periodontal pocket; (2) controlled-release devices can maintain these high concentrations for an extended period; and (3) complications associated with systemic administration are generally reduced [
15].
In the present study, no treatment-related adverse effects were observed in any patients. In contrast, Garrett et al. [
29] reported mild gingival soreness as one of the findings in patients treated with tetracycline LDD, and Polson et al. [
30] reported abscess formation and occurrence of oral candidiasis during their study period. There was gradual reduction in PI scores throughout the study period, which was statistically highly significant (
P<0.001), similar to the results of Drisko et al. [
31], Mombelli et al. [
3], and Polson et al. [
30]. A statistically highly significant (
P<0.001) reduction in GI scores was also observed, which was similar to the results of Friesen et al. [
32] and Heijl et al. [
33]. There was a highly significant improvement of SBI at various time intervals of the study period, which was consistent with the findings of Heijl et al. [
33] and Friesen et al. [
32]. The gradual improvements in the reduction of PI, GI, and SBI were attributed to the reinforcement of OHI during the recall visits of the study period. Group II (SRP) sites showed a highly significant reduction in PD scores, which was similar to the findings of Drisko et al. [
31] and Heijl et al. [
33]. On comparing PD scores between different time intervals, we found a significant reduction between D0 and D15 and between D0 and D45, which was similar to the findings of Friesen et al. [
32]. However, between D15 and D45, no significant difference in the PD scores was found. Group III (SRP with LDD) sites showed a highly significant reduction (
P<0.001) in PD scores during the study period. This finding was similar to that of Drisko et al. [
31], Heijl et al. [
33], and Mombelli et al. [
3]. On comparing PD scores between different time intervals, we found a significant reduction (
P<0.05) between D0 and D15 and between D0 and D45. These findings were similar to those of Friesen et al. [
32] and Polson et al. [
30]. However, between D15 and D45, there was no significant difference in the PD scores. On comparing groups II and III sites, we found that the variation in PD scores was not significant between different intervals of the study period; which was consistent with the findings of Heijl et al. [
33] and Friesen et al. [
32]. This effect may be attributed to the crossover effect of split mouth design of our study. However, Drisko et al. [
31] found a statistically significant PD reduction between two groups, but the difference found was very small and not clinically significant.
Group II sites showed a significant gain (
P<0.05) in CAL, which is consistent with the findings of Polson et al. [
30], Friesen et al. [
32], and Drisko et al. [
31]. In contrast, Eckles et al. [
34] found no significant gain (
P<0.05) in CAL over a period of time. On comparing CAL scores between different time intervals, we found a significant gain between D0 and D15 and between D0 and D45, which is similar to the findings of Goodson et al. [
35]. However, Eckles et al. [
34] and Friesen et al. [
32] showed no significant gain in the CAL score among different intervals of their study period. The clinical attachment gain was not significant between D15 and D45. Group III sites showed a highly significant gain (
P<0.001) in the CAL, which is similar to the findings of Friesen et al. [
32], Polson et al. [
30], and Mombelli et al. [
3]. On comparing CAL scores among different time intervals, we found a significant improvement between D0 and D15 and between D0 and D45. These results are similar to those of Drisko et al. [
31]. In contrast, Eckles et al. [
34] and Friesen et al. [
32] found no significant difference in CAL scores over a period of time. The clinical attachment gain was not significant between D15 and D45 (
P<0.05). Upon comparing groups II and III sites, we found that the variation in CAL scores was significant between D0 and D15 and between D0 and D45, which is similar to the findings of Goodson et al. [
35] and Friesen et al. [
32]. On the other hand, Drisko et al. [
31] and Eckles et al. [
34] showed no significant difference in CAL scores between two treatment groups. However, upon comparing CAL scores between D15 and D45, we found that there was no significant difference.
In the present study, no GR was seen at any of the sites. This may be due to the shorter duration of the study period and non-inclusion of deeper pockets (>6 mm). However, Mombelli et al. [
3] reported a recession of 0.64 mm, while Tonetti et al. [
36] reported a recession of 2 mm during their study period.
In most of the studies, GCF leptin levels were compared between different individuals, and a greater reduction in GCF leptin levels for patients with periodontitis than for periodontally healthy subjects was found [
5,
9,
10]. In our study, there was no significant difference in the GCF leptin levels among the study group sites at D0, D15, and D45. This may be due to the split mouth study design resulting in a serum leptin effect at all the selected sites in the oral cavity during the study period. Group II sites showed no significant change in GCF leptin, while group III showed a significant change (
P<0.05) at different time intervals. Between D0 and D15 and between D15 and D45, GCF leptin levels of group III sites showed significant variation (
P<0.05).
To the best of our knowledge, there is no scientific literature to support the effect of SRP and SRP with LDD on the GCF leptin level.
At baseline, though nonsignificant, we observed higher mean GCF leptin values at periodontitis sites (groups II and III) than at the healthy sites (group I), which contradicts the results of Karthikeyan and Pradeep [
9,
10]. This variation in result may be attributed to the use of different study designs. According to Sorensen et al. [
37], the serum leptin level depends more precisely on the process of fat accumulation in the adipocytes than on the overall amount of stored fat. Thus, BMI is not a true indicator of the total fat mass and the distribution of fat mass. The present study utilized a split mouth design; hence, all factors related to fat distribution, total fat mass, and size of adipocytes remain unchanged for all the sites belonging to groups I, II, and III. As OHI reinforcement was done at recall visits (D15 and D45), it was assumed that the GCF leptin levels would remain constant at control sites throughout the study period; hence, no GCF samples were collected. This limits our study with respect to an intergroup comparison of GCF leptin.
We observed the reduction in GCF leptin values between D0 and D15 for groups II and III sites, following which there was re-elevation between D15 and D45. The amount of reduction in the GCF leptin level was higher in group III than in group II sites. A comparison of GCF leptin levels at different time intervals revealed that group III sites showed a statistically significant variation between D0 and D15 and between D15 and D45.
Various authors (Johnson and Serio [
5] and Bozkurt et al. [
24]) have suggested the possible role of leptin receptor in the variations seen in leptin levels. Ge et al. [
38] identified cellular isoforms of leptin receptors. A soluble leptin receptor (SLR) is generated by matrix metalloproteinase-mediated shedding of the leptin receptor ectodomain. Recently, it has been suggested that leptin plays a significant role in bone formation. Leptin at a high local concentration protects the host from inflammation and infection and maintains the bone level [
39,
40]. Detection of leptin receptors and their role in periodontal tissues has not been pursued thus far. As our study shows, on the basis of the initial reduction in leptin levels following treatment with subsequent re-elevation, it may be hypothesized that the presence of various leptin receptors and SLR in periodontal tissues may be responsible for the obtained inconsistent results.
In conclusion, in the present study, there was progressive improvement in clinical parameters after periodontal therapy with a more significant reduction in leptin in group III than in group II sites, following which there was re-elevation of GCF leptin to almost the pretreatment level, which suggested that leptin not only mediated periodontal local inflammation but also had systemic effects. There was no correlation between the clinical parameter scores and the GCF leptin level. This may suggest that GCF leptin is not only influenced by the health status of periodontal tissue but also by the health status of other body tissues like adipose tissue. Leptin also regulates the production of various proinflammatory cytokines such as tumor necrosis factor alpha, IL-1, and IL-6. Hence, the levels of leptin in GCF can be used as a predictive biomarker for the severity of chronic periodontitis. Further long-term multicenter trial studies are necessary to evaluate the possible correlation of GCF and serum leptin levels with radiographic bone changes in various periodontal diseases and systemic conditions. Existence of leptin receptors in periodontal tissues and their effect on various periodontal diseases need to be evaluated in order to understand the role of leptin in periodontal health and disease. In addition, the possible effect of periodontal therapy on serum leptin levels needs further evaluation.




 PDF
PDF ePub
ePub Citation
Citation Print
Print








 XML Download
XML Download