Abstract
Purpose
This in vitro study was performed to assess the adherence of Porphyromonas gingivalis to a resorbable blast media (RBM) titanium surface pretreated with an ultrasonic scaler or toothbrush and to evaluate the effects of the treatment of the RBM titanium discs on the bacterial removal efficiency of brushing by crystal violet assay and scanning electron microscopy.
Methods
RBM titanium discs were pretreated with one of several ultrasonic scaler tips or cleaned with a toothbrush. Then the titanium discs were incubated with P. gingivalis and the quantity of adherent bacteria was compared. The disc surfaces incubated with bacteria were brushed with a toothbrush with dentifrice. Bacteria remaining on the disc surfaces were quantified.
Results
A change in morphology of the surface of the RBM titanium discs after different treatments was noted. There were no significant differences in the adherence of bacteria on the pretreated discs according to the treatment modality. Pretreatment with various instruments did not produce significant differences in the bacterial removal efficiency of brushing with dentifrice.
Titanium is recognized for its excellent biocompatibility in many applications, including dental and hip implants [1], and osseointegrated titanium dental implants have played an important role in restoring the missing dentition [2]. However, infections do occur, exposing the implant threads to oral microorganisms, resulting in bacteria-induced peri-implant tissue destruction [3]. Once the implant surface is exposed to the oral cavity, it is immediately covered by a salivary pellicle and colonized by microorganisms [4]. Mechanical techniques including scaling with metal, plastic, or ultrasonic instruments; air-powder abrasive; rubber cup polishing; and brushing with a conventional or rotating brush have been applied for the debridement of dental implants [3].
In recent years, surface treatments have been performed on machined titanium implants to improve osseointegration [5,6]. The resorbable blast media (RBM) surface is prepared by sandblasting machined titanium with calcium phosphate ceramic media or hydroxyapatite particle-containing blast media [7,8]. The roughened RBM titanium surfaces exhibited better early cell attachment of osteoblast-like MG-63 cells than the smooth surfaces in the presence of serum [9], and the osteoblast-like MG-63 cells cultured on the RBM surfaces produced a greater amount of alkaline phosphatase than the cells cultured on smooth surfaces [10]. A statistically significant increase was found in the bone-to-implant contact percentages in the RBM implants compared to the machined surface fixtures [11]. The overall implant success rate of RBM implants was 99.3% in the nongrafted mandible and 100% for the nongrafted maxilla without any discernible crestal bone loss [12]. A similar result was obtained for grafted jaws, with a survival rate of 98.7% [7]. However, these rough surfaces are reported to favor colonization by bacteria, and the development of peri-implantitis is more likely when compared with smooth surfaces [4,13,14].
Instrumentation with various instruments may cause changes in the titanium surface [15-17]. In some studies, the use of an ultrasonic metal tip produced substantial changes when compared with nonmetal tips [16], but no significant differences were reported in other studies comparing the metal scaler tips to the plastic tips [18]. Surface morphology alterations may lead to changes in the properties that may influence the bacterial adhesion and the removal of biofilms. However, the effects of previous instrumentation on the efficiency of bacterial removal by oral hygiene measures have rarely been examined.
Therefore, this in vitro study was performed (1) to assess the adherence of Porphyromonas gingivalis on the RBM titanium surface pretreated with an ultrasonic scaler and toothbrush and (2) to evaluate the effects of treatment of RBM titanium discs on the bacterial removal efficiency of brushing using a crystal violet assay and scanning electron microscopy (SEM).
Fig. 1 shows an overview of the study design.
Titanium discs treated with RBM (Neobiotech, Co., Ltd, Seoul, Korea ), measuring 9 mm in diameter and 3 mm in thickness were used in this study. The effect of instrumentation with several metal and nonmetal tips from two different manufacturers and brushing with dentifrice on the adherence of P. gingivalis and efficiency of bacterial removal by brushing were evaluated. The discs were divided into six groups: (1) no treatment, (2) ultrasonic scaler with metal tip (A-M) (PS, Mini Piezon, Electro Medical Systems, Nyon, Switzerland), (3) ultrasonic scaler with plastic tip (A-P) (Peek tip, Electro Medical Systems), (4) ultrasonic scaler with metal tip (B-M) (1, Satelec, Suprasson, La Ciotat, France), (5) ultrasonic scaler with carbon tip (B-C) (PH1, Satelec), and (6) toothbrush (implant care brush; Br) (Implant Care, TePe, Malmö, Sweden) (Fig. 2). Three RBM titanium discs were used for each group per experiment. The whole of the top surface of the titanium discs was instrumented for a total of 40 strokes by a single operator (Y.K.). The scaler tip was angled tangentially and care was taken to apply approximately 30 g of pressure. Back and forth movement was performed in the same direction for 40 strokes. A power setting of 3 was applied for the A-M and A-P groups and the B-M and B-P groups were used in mode P, with a power setting of 3. For the brushing group (group 6), the surface was brushed with the dentifrice (Anti-Plaque, Bukwang, Seoul, Korea) that contained fluoride for 20 seconds. Then the discs were rinsed with tap water and brushed again for 20 seconds, making the total brushing time 40 seconds, and the discs were rinsed with tap water again afterwards.
Pretreated RBM discs were placed one in each well of a 24-well plate, and 1 mL of culture medium (brain heart infusion, BD, Franklin Lakes, NJ, USA) supplemented with 10 µg/mL hemin (Sigma-Aldrich Co., St. Louis, MO, USA) and 0.2 µg/mL vitamin K (Sigma-Aldrich Co.) was added to each well. P. gingivalis (ATCC 33277, American Type Culture Collection, Manassas, VA, USA) was inoculated at 2×108/mL and was incubated anaerobically in an atmosphere of 85% N2, 10% H2, and 5% CO2 at 37℃ for 2-3 days.
Following incubation of the pretreated titanium discs with P. gingivalis, the discs were washed with phosphate buffered saline (PBS) twice to remove unattached bacteria and debris. Each disc was fixed with 2.5% glutaraldehyde in PBS overnight. The discs were then washed in PBS three times and postfixed in 1% osmium tetroxide for 1.5 hours. The samples were then dehydrated through a graded series of ethanol (70%, 80%, 90%, and 95% for 15 minutes, and 100% twice for 15 minutes) and mounted on stubs. The bacteria were then air-dried by evaporation of hexamethyldisilazane on a clean bench, sputter coated with gold-palladium, and observed using a scanning electron microscope (S-4700, Hitachi, Tokyo, Japan) at 15 kV at a magnification of ×5,000.
Three discs in each group were used for the crystal violet assay. The crystal violet assay was performed to evaluate the total amount of bacteria on the pretreated RBM surface. The adhered bacteria were stained with 1% crystal violet for 10 minutes at room temperature, and water spray from the ultrasonic scaler used on group 2 (A-M) was used to remove unbound dye. The ultrasonic scaler tip was held close to the disc surface, but the tip was not allowed to contact the disc surface. The bound dye was extracted using destaining solution consisting of 80% ethanol and 20% acetone. The amount of bacteria was measured at an optical density of 570 nm using a microplate reader (BioTek, Winooski, VT, USA). The optical density was measured in duplicate.
The discs were treated with various instruments and bacteria were grown on the pretreated surfaces, as previously described. Then the top surface of the discs from groups 1-6 was brushed for total of 40 seconds (20 seconds, two cycles) with a toothbrush (Implant Care) and dentifrice (Anti-Plaque).
The crystal violet assay was performed to quantitatively evaluate the bacterial removal efficiency after brushing. The assay was performed as described above.
Following brushing of the pretreated titanium discs, the surfaces were washed with PBS twice to remove debris. The surfaces were evaluated to determine the efficiency of bacterial removal by the brush using SEM according to the protocol described above. Two discs were used for each group, and ten images were randomly captured from each disc at a magnification of ×5,000. The images were saved as tiff files and the bacteria remaining on the disc were manually counted. If a whole bacterium was not visible in the image or if the bacterium was touching the margin, it was not counted.
Data were represented as mean±standard deviation. One-way analysis of variance was used to test for differences between treatment groups with commercially available statistical software IBM SPSS ver. 19.0 (IBM Co., Armonk, NY, USA). Statistically significant differences were evaluated with the significance set at P<0.05.
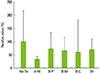
The gross morphology of the surface of the RBM titanium discs after different treatments including various ultrasonic scaler tips and brushing is shown in Fig. 3. Differences in surface changes among the groups could be discerned with the naked eye. The change in the surfaces was more obvious when the RBM surfaces were treated with metal tips, including both the A-M and B-M groups. Multiple layers of bacterial colonization could be seen on all the discs. The relative amount of adhering bacteria after incubation on each of the pretreated surfaces is presented in Fig. 4. The A-M, A-P, B-M, B-C, and Br groups had 107.8%±27.7%, 113.7%±22.4%, 105.7%±29.7%, 82.8%±43.5%, and 105.8%±68.0% of the bacteria relative to the amount on the surface of the no treatment group (Fig. 5). The amount of P. gingivalis biofilm formed on the discs after treatment was similar irrespective of the treatment method used (P>0.05). Previous instrumentation with an ultrasonic scaler and a toothbrush on the implant surface did not seem to influence the adherence of bacteria at 48 hours.
The gross morphology of the surface of the pretreated titanium discs after additional brushing can be seen in Fig. 6. The color of the disc surface treated with the carbon tip seems to be less white after brushing with dentifrice (Fig. 6E). Some remnants of dentifrice can be seen on the rim of the disc (Fig. 6F). No additional changes in the surfaces of the other discs could be seen with the naked eye after brushing. Brushing decreased the amount of bacteria adhering on the disc surfaces in all of the discs. This can be observed in Fig. 7, which shows the surface of the discs observed with SEM microscopy at ×5,000. Single bacterial cells that are adhering to the surfaces can be observed and counted. P. gingivalis are seen in the crevices and fissures of the rough surface but they can also be seen remaining even on the smoothened surfaces. The relative value of the remaining bacteria on the pretreated RBM titanium surface with brushing determined by crystal violet assay is shown in Fig. 8. The A-M, A-P, B-M, B-C, and Br groups had 33.1%±11.5%, 72.6%±61.7%, 65.9%±49.6%, 60.5%±121.0%, and 69.5%±39.4% of bacteria, respectively, relative to the amount of bacteria remaining on the no treatment group (control) surface. These results showed that brushing of the A-M surface exhibited the highest bacterial removal efficiency; however, these differences between the groups were not statistically significant (P>0.05). The average number of adhering bacteria in each group was compared using SEM images. The no treatment, A-M, A-P, B-M, B-C, and Br groups had 2.8%±4.2%, 0.5%±1.1%, 2.9%±3.2%, 8.2%±11.1%, 1.3%±2.5%, and 1.7%±2.5% of bacteria relative to the amount of bacteria remaining on the no treatment group, respectively. Similarly, the A-M surface exhibited the highest bacterial removal efficiency, but this did not reach statistical significance (P>0.05).
This in vitro study was done to evaluate the effect of instrumentation on the adherence of bacteria to RBM titanium discs and to elucidate the effects of pretreatment of the titanium on the bacterial removal efficiency of brushing with dentifrice.
Adherence of bacteria was evaluated after treating the titanium surface up to 40 seconds with various instruments. SEM evaluation revealed that instrumentation of the titanium surface did not have any influence on the colonization of the bacteria after 2-3 days. Moreover, the quantitative data showed that the adherence of bacteria on the pretreated titanium surface was similar regardless of the treatment modality, even though the surface roughness of the discs after treatment with various instruments differed.
The removal of biofilms from microstructured titanium used for dental implants is still an unresolved challenge [19]. In this study, the removal of bacteria was evaluated after brushing the pretreated RBM titanium surfaces for 40 seconds. The SEM images showed that bacteria was still residing in the pits of the titanium surfaces, and it may be difficult to completely remove the bacteria from these deep niches [20]. In this respect, treatment that leads to reduced roughness may have benefits for the ease of maintenance of implants. Treatment with a metal tip (A-M group) showed reduction of the roughness value in a previous report [21], and the highest bacterial removal efficiency within the ultrasonic groups was achieved in this group (A-M). Thus, our group has suggested that this may be considered a treatment option for RBM surfaces. Likewise, the lowest amount of remaining bacteria was seen in the A-M group; however, this difference was not statistically significant. All of the treated surfaces contained areas that were uninstrumented, and this may have influenced the results. The amount of bacteria in the deep pits may have been great enough to mask the differences in bacterial adhesion on the treated areas. In this experiment, the number of strokes used for instrumentation was standardized, but this method caused the total area of the surface that had been treated to differ among the samples. As the contact angle of the various tips differs, the use of the plastic tips showed a greater treated area. This may have led to the fact that the amount of bacteria remaining after brushing did not significantly differ according to the pretreatment method.
The bristles of toothbrushes from various manufacturers come in various widths and configurations. A study on the effects of different type of bristles regarding the efficacy of access to occlusal fissures showed that a combination of tapered and round-end bristles was statistically significantly more effective in removing the artificial plaque material on the fissure area than the rounded-end bristle group [22]. The design of toothbrushes with different configurations of bristles (extended, x-angled, or flat multitufted bristles) is reported to influence the access efficacy, and a manual toothbrush with extended bristles showed the greatest clinical interproximal plaque removal [23].
Controversy exists regarding the abrasiveness of fluoride toothpastes [24]. When commercially pure titanium was brushed with one of four toothpastes of different relative dentin abrasivities, no significant differences were found in the titanium roughness [25]. However, another study showed that the use of dentifrice during bushing affected the topography and roughness of titanium surfaces [24]. Other researchers have reported that alterations in surface morphology may affect the biological responses of the titanium in the oral environment and the use of a dentifrice with lower abrasivity might be advisable for the daily oral hygiene practices of patients with titanium dental devices [26].
The titanium surface may be influenced by the use of fluoride-containing dentifrice. In some research, the presence of fluoride was unfavorable for the stability of the titanium [1]. However, another report showed that dentifrice with a low ionizable fluoride content (0.125%) used in brushing the natural teeth did not cause deterioration of titanium abutments [27]. The acidity of the dentifrice may influence the results. Irrespective of the dentifrice, neutral slurries, like alkaline slurries, yielded a rough texture, whereas acidic slurries yielded a relatively smooth texture [28]. It was also observed that an acidic slurry-induced smooth surface may minimize plaque formation.
Overall, this study showed that various types of mechanical instrumentation influenced the surface of a RBM titanium surface but did not influence the adherence of bacteria to that surface at 2-3 days or the cleaning efficiency of brushing. Further studies are needed to evaluate the effects of different types of bristles and dentifrices on titanium surfaces. Whether the type of mechanical instrumentation used influences early bacterial adhesion and biofilm formation should be evaluated also.
Figures and Tables
Figure 1
Overview of the study design. RBM: resorbable blast media, SEM: scanning electron microscopy.
Figure 2
Metal and nonmetal ultrasonic scaler tips and toothbrush. (A) Ultrasonic scaler with metal tip (PS, Electro Medical Systems, Nyon, Switzerland), (B) ultrasonic scaler with plastic tip (PEEK tip, Electro Medical Systems), (C) ultrasonic scaler with metal tip (1, Satelec, Suprasson, La Ciotat, France), (D) ultrasonic scaler with carbon tip (PH1, Satelec), and (E) toothbrush (Implant Care, TePe, Malmö, Sweden).

Figure 3
The gross morphology of the untreated and treated resorbable blast media surfaces of the titanium discs. (A) No treatment, (B) A-metal, (C) A-plastic, (D) B-metal, (E) B-carbon, and (F) brush.

Figure 4
Bacteria cultured on the treated surfaces were examined with scanning electron microscopy at ×5,000. (A) No treatment, (B) A-metal, (C) A-plastic, (D) B-metal, (E) B-carbon, and (F) brush.

Figure 5
Relative value of the amount of bacteria on the resorbable blast media disc surfaces compared by crystal violet assay. The amount of bacteria on the untreated group was considered to be 100%. Tx: treatment, A-M: A-metal, A-P: A-plastic, B-M: B-metal, B-C: B-carbon, and Br: brush.

Figure 6
The gross morphology of the pretreated RBM titanium surfaces after brushing with dentifrice. (A) No treatment, (B) A-metal, (C) A-plastic, (D) B-metal, (E) B-carbon, and (F) brush.

Figure 7
The bacteria were grown on pretreated surfaces, and all of the surfaces were brushed with dentifrice. The figure shows the surface morphology using scanning electron microscopy. (A) No treatment, (B) A-metal, (C) A-plastic, (D) B-metal, (E) B-carbon, and (F) brush.
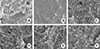
Figure 8
Relative value of the remaining bacteria on the pretreated RBM titanium surface after brushing by crystal violet assay. The amount of remaining bacteria on the untreated group was considered to be 100%. Tx: treatment, A-M: A-metal, A-P: A-plastic, B-M: B-metal, B-C: B-carbon, and Br: brush.
ACKNOWLEDGEMENTS
This research was supported by the 2013 Seoul St. Mary's Hospital Clinical Medicine Research Program through the Catholic University of Korea. The authors acknowledge Neobiotech Co., Ltd. (Seoul, Korea) for donating the titanium discs for this study.
References
1. Lindholm-Sethson B, Ardlin BI. Effects of pH and fluoride concentration on the corrosion of titanium. J Biomed Mater Res A. 2008; 86:149–159.

2. Groessner-Schreiber B, Hannig M, Duck A, Griepentrog M, Wenderoth DF. Do different implant surfaces exposed in the oral cavity of humans show different biofilm compositions and activities? Eur J Oral Sci. 2004; 112:516–522.

3. Alhag M, Renvert S, Polyzois I, Claffey N. Re-osseointegration on rough implant surfaces previously coated with bacterial biofilm: an experimental study in the dog. Clin Oral Implants Res. 2008; 19:182–187.

4. Baffone W, Sorgente G, Campana R, Patrone V, Sisti D, Falcioni T. Comparative effect of chlorhexidine and some mouthrinses on bacterial biofilm formation on titanium surface. Curr Microbiol. 2011; 62:445–451.

5. Cooper LF. A role for surface topography in creating and maintaining bone at titanium endosseous implants. J Prosthet Dent. 2000; 84:522–534.

6. Elias CN, Meirelles L. Improving osseointegration of dental implants. Expert Rev Med Devices. 2010; 7:241–256.

7. Franco M, Rigo L, Viscione A, De Santis B, Tropina E, Brunelli G, et al. CaPO4 blasted implants inserted into iliac crest homologue frozen grafts. J Oral Implantol. 2009; 35:176–180.

8. Sanz A, Oyarzun A, Farias D, Diaz I. Experimental study of bone response to a new surface treatment of endosseous titanium implants. Implant Dent. 2001; 10:126–131.

9. Nishimoto SK, Nishimoto M, Park SW, Lee KM, Kim HS, Koh JT, et al. The effect of titanium surface roughening on protein absorption, cell attachment, and cell spreading. Int J Oral Maxillofac Implants. 2008; 23:675–680.
10. Pae A, Kim SS, Kim HS, Woo YH. Osteoblast-like cell attachment and proliferation on turned, blasted, and anodized titanium surfaces. Int J Oral Maxillofac Implants. 2011; 26:475–481.
11. Piattelli M, Scarano A, Paolantonio M, Iezzi G, Petrone G, Piattelli A. Bone response to machined and resorbable blast material titanium implants: an experimental study in rabbits. J Oral Implantol. 2002; 28:2–8.

12. Gonshor A, Goveia G, Sotirakis E. A prospective, multicenter, 4-year study of the ACE Surgical resorbable blast media implant. J Oral Implantol. 2003; 29:174–180.

13. Badihi Hauslich L, Sela MN, Steinberg D, Rosen G, Kohavi D. The adhesion of oral bacteria to modified titanium surfaces: role of plasma proteins and electrostatic forces. Clin Oral Implants Res. 2013; 24:Suppl A100. 49–56.

14. Drake DR, Paul J, Keller JC. Primary bacterial colonization of implant surfaces. Int J Oral Maxillofac Implants. 1999; 14:226–232.
15. Takasaki AA, Aoki A, Mizutani K, Kikuchi S, Oda S, Ishikawa I. Er:YAG laser therapy for peri-implant infection: a histological study. Lasers Med Sci. 2007; 22:143–157.

16. Kawashima H, Sato S, Kishida M, Yagi H, Matsumoto K, Ito K. Treatment of titanium dental implants with three piezoelectric ultrasonic scalers: an in vivo study. J Periodontol. 2007; 78:1689–1694.

17. Park JB, Kim N, Ko Y. Effects of ultrasonic scaler tips and toothbrush on titanium disc surfaces evaluated with confocal microscopy. J Craniofac Surg. 2012; 23:1552–1558.

18. Sato S, Kishida M, Ito K. The comparative effect of ultrasonic scalers on titanium surfaces: an in vitro study. J Periodontol. 2004; 75:1269–1273.

19. Rupf S, Idlibi AN, Marrawi FA, Hannig M, Schubert A, von Mueller L, et al. Removing biofilms from microstructured titanium ex vivo: a novel approach using atmospheric plasma technology. PLoS One. 2011; 6:e25893.

20. Pereira da Silva CH, Vidigal GM Jr, de Uzeda M, de Almeida Soares G. Influence of titanium surface roughness on attachment of Streptococcus sanguis: an in vitro study. Implant Dent. 2005; 14:88–93.

21. Park JB, Jang YJ, Koh M, Choi BK, Kim KK, Ko Y. In vitro analysis of the efficacy of ultrasonic scalers and a toothbrush for removing bacteria from resorbable blast material titanium disks. J Periodontol. 2013; 84:1191–1198.

22. Hotta M, Sekine I, Imade S, Sano A. Evaluation of tapered-end toothbrush bristles regarding efficacy of access to occlusal fissures. J Clin Dent. 2002; 13:225–227.
23. Stiller S, Bosma ML, Shi X, Spirgel CM, Yankell SL. Interproximal access efficacy of three manual toothbrushes with extended, x-angled or flat multitufted bristles. Int J Dent Hyg. 2010; 8:244–248.

24. Fais LM, Fernandes-Filho RB, Pereira-da-Silva MA, Vaz LG, Adabo GL. Titanium surface topography after brushing with fluoride and fluoride-free toothpaste simulating 10 years of use. J Dent. 2012; 40:265–275.

25. Molina C, Nogues L, Martinez-Gomis J, Peraire M, Salsench J, Sevilla P, et al. Dental casting alloys behaviour during power toothbrushing with toothpastes of various abrasivities. Part II: corrosion and ion release. J Mater Sci Mater Med. 2008; 19:3015–3019.

26. Hossain A, Okawa S, Miyakawa O. Effect of toothbrushing on titanium surface: an approach to understanding surface properties of brushed titanium. Dent Mater. 2006; 22:346–352.

27. Siirila HS, Kononen M. The effect of oral topical fluorides on the surface of commercially pure titanium. Int J Oral Maxillofac Implants. 1991; 6:50–54.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download