Abstract
Purpose
Matrix metalloproteinases (MMPs) are capable of degrading extracellular matrix, and they are inducible enzymes depending on an inflammatory environment such as periodontitis and bacterial infection in periodontal tissue. Gingival inflammation has been postulated to be correlated with the production of MMP-2 and MMP-9. The objective of this study was to quantify the expression and activity of MMP-9 and -2, and to determine the correlation between activity and expression of these MMPs in human gingival tissues with periodontitis.
Methods
The gingival tissues of 13 patients were homogenized in 500 µL of phosphate buffered saline with a protease inhibitor cocktail. The expression and activity of MMP-2 and -9 were measured by enzyme-linked immunosorbent assay and Western blot analysis, and quantified by a densitometer. For the correlation line, statistical analysis was performed using the Systat software package.
The main factor jeopardizing the longevity of a tooth in adults is periodontal inflammation, and one of the characteristics of this pathology is alveolar bone loss [1]. It has been reported that some loss of periodontal attachment and alveolar bone is to be anticipated in older patients because of a critical loss of periodontal supporting tissues [2,3]. Proteases might play a prominent role in cell migration and wound healing, and their expression is induced by treatment of periodontal cells with tumor necrosis factor-α (TNF-α) or interleukin (IL)-1β [4]. These mediators directly or indirectly participate in tissue destruction and bone resorption [5-7]. The matrix metalloproteinases (MMPs) are a group of enzymes thought to be important in this degenerative process [8,9]. MMPs can be released from inflammatory cells recruited by bacterial infection and gingival fibroblasts in response to cytokines derived from these inflammatory cells in periodontal patients [10,11].
MMPs are a family of zinc- and calcium-dependent endoproteinases that mediate the degradation of extracellular matrix (ECM) and basement membrane components [12]. This group of 23 human enzymes is classified into collagenases, gelatinase, stromelysins, membrane-type MMPs, and other MMPs, mainly based on the substrate specificity and molecular structure [13]. Among these MMPs, MMP-1, -2, -3, -8, and -9 have been found in human inflammatory periodontal tissues [14]. In particular, MMP-2 and -9 are known specifically to cleave type IV collagen, which is a major structural component of basement membrane [15,16]. MMP-2 is secreted by gingival fibroblasts and MMP-9 is mainly secreted by polymorphonuclear leukocytes [14,17].
The objective of this study was to quantify the expression and activity of MMP-9 and -2, and to determine the correlation between the activity and expression of these MMPs in human gingival tissues with periodontitis.
The study employed gingival tissues of 13 human subjects with periodontal disease, aged 31 to 70 years old (mean, 52.4 years), after the study proposal was approved by the IRB of the Kyung Hee University Dental Hospital (Reg. No. 2009-2, June 29, 2009). The donor patients voluntarily signed written informed consent, and accordingly, the samples were taken during periodontal surgery in the course of normal treatment.
Gingival tissue samples of 13 patients were each homogenized in 500 µL of phosphate buffered saline (137 mM NaCl, 10 mM Na2HPO4, and 2.7 mM KCl, pH 7.3) with protease inhibitor cocktail (Roche Korea, Seoul, Korea). The samples were centrifuged at 13,000 rpm for 15 minutes at 4℃, and the supernatant was used to quantify the amount of protein present. Protein concentrations were measured using the Bio-Rad protein assay (Bio-Rad Laboratories Inc., Hercules, CA, USA).
The anti-MMP-9 and -2 (Chemicon International, Temecula, CA, USA) and goat antirabbit immunoglobulin G-horse-radish peroxidase (HRP) (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) were purchased for this study. Protein concentrations were measured using the Bio-Rad protein assay (Bio-Rad Laboratories Inc.). One hundred milligrams protein samples were boiled in sample buffer (60 mM Tris-Cl [pH 6.8], 25% glycerol, 2% sodium dodecyl sulfate [SDS], 14.4 mM 2-mercaptoethanol, 0.1% bromophenol blue). The prepared samples were separated by 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and electrophoretically transferred onto nitrocellulose transfer membrane (Whatman Inc., Sanford, ME, USA). The membranes were subsequently blocked with blocking solution containing 5% skim milk in Tris-buffered saline-Tween 20 (TBST) (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, and 0.1% Tween 20) for 1 hour, and then immune-blotted with 1:1,000 diluted anti-MMP-9 and -2 antibodies (Chemicon International) for 12 hours at 4℃. After washing with TBST three times for 10 minutes each, the membranes were incubated with secondary antirabbit HRP-conjugated antibodies (Santa Cruz Biotechnology Inc.). After washing with TBST three times for 10 minutes each, the bands were visualized by an enhanced chemiluminescence system (Mbiotech, Guri, Korea). Quantification of MMP was performed using a Multi Gauge densitometry program (Fujifilm, Seoul, Korea).
The enzyme activity of MMP-2 and -9 were measured by a zymogram protease assay. Briefly, 10% SDS-PAGE gel, containing 0.1% gelatin (Sigma-Aldrich Co., St. Louis, MO, USA) as the substrate was used for electrophoresis. Protein concentrations were measured using the Bio-Rad Protein assay (Bio-Rad Laboratories Inc.). Fifteen milligrams protein samples were mixed in zymo-sample buffer (60 mM Tris-Cl [pH 6.8], 25% glycerol, 2% SDS, and 0.1% bromophenol blue). The prepared samples were separated by 10% SDS-PAGE at 4℃. After electrophoresis, the gel was washed twice with 2.5% Triton X-100 (Sigma-Aldrich Co.) on a shaker for 15 minutes to remove SDS, and washed twice with water. The gel was then incubated in reaction buffer (50 mM Tris-HCl, pH 8.0, 200 mM NaCl, 5 mM CaCl2, 0.2% Brij35) at 37℃. After 20 hours of incubation, the gel was stained with Coomasie Blue staining solution (0.1% Coomassie Brilliant Blue R-250, 50% methanol, and 10% glacial acetic acid) for 1 hour and then destained with destaining solution (10% acetic acid and 20% methanol in water). Gelatinolytic activity was manifested as horizontal white bands on a dark blue background. The gels were scanned. Quantification of MMP activity was performed using the Multi Gauge densitometry program (Fujifilm).
The author employed human gingival tissue samples from 13 patients with periodontitis. To analyze the expression level of MMP-9 and -2, Western blot analysis was performed using the respective antibodies. The expression levels of MMP-9 and -2 in each sample were quantified by a densitometer, and are shown in Fig. 1 and Table 1. MMP-9 was highly expressed in all gingival tissues, whereas the expression of MMP-2 was shown to be relatively lower than that of MMP-9.
To analyze the activity of MMP-9 and -2, a gelatin zymography assay was performed. Gelatin zymography data from 13 samples showed variation by the individual. As shown in Fig. 2, MMP-9, corresponding to molecular weight 94 kDa, showed generally high activity levels, except in patient numbers 1, 3, 4, and 8. In particular, patient numbers 2 and 7 had very high levels of MMP-9 activity (Fig. 2). MMP-2, corresponding to molecular weight 72 kDa, showed a moderate activity level and MMP-2 activity levels were lower than those of MMP-9, as evidenced by the Western blot (Fig. 2).
The overall results illustrated that the expression and activity of MMP-9 and -2 manifested wide variation within an identical sample. Each subject represented a different correlation between expression and activity of MMP-9 and -2. As shown in Fig. 3, MMP-9 activity increased together with the MMP-9 expression level, exhibiting a positive correlation (r=0.793, P=0.01), while this association was not observed in MMP-2.
The family of MMPs, composed of 23 related zinc- and calcium-dependent endopeptidases are derived predominantly from polymorphonuclear leukocytes during the acute stages of periodontal disease and are key enzymes responsible for extracellular collagen matrix degradation [17,18]. The MMP family has been classified into three groups by the enzyme substrate specificity of the interstitial collagenases, the gelatinases (type IV collagenases), and the stromelysins [19]. Among them, the gelatinases, or type IV collagenases, are closely related in their structure and enzyme action on substrates and they exist in a 72-kDa form (MMP-2) and a 92-kDa form (MMP-9). MMP-9 is expressed from macrophages, connective tissue cells, and tumors. The MMP-2 and -9 enzymes are crucial for extracellular degradation of denatured collagens and degradation of extracellular basement membrane type IV collagen and related matrix components [19].
A critical outcome of periodontal diseases is the degradation of the collagenous structure of periodontal tissues, and MMPs have been implicated in the progression of this disease. As the periodontal lesion develops, the junctional epithelium migrates apically, leading to loss of attachment. This process requires not only cell proliferation, but also migration of the cells over the tooth and connective tissue substratum that has been modified by the inflammatory process. Previous reports have demonstrated that gingival epithelial cells express several MMPs in inflamed periodontal tissues, including MMP-2, -3, -8, and -13 [18]. Normal growth and development are based on oral connective tissue remodeling and oral periodontal diseases are linked with the breakdown of the collagen-based bone matrix, bone cartilage, and surrounding tissues. MMPs are well known to play a key role in bone matrix degradation [19]. In addition, elevated MMP levels have been observed in inflamed human gingiva and gingival crevicular fluid in periodontitis patients [17].
The relationship between MMPs and the loss of connective tissue has been well demonstrated by recent studies that were performed using chronically inflamed gingival tissues. Until now, different types of MMPs including MMP-1, -2, -3, -7, -8, -9, -13, -14, -25, and -26 have been observed in human gingival biopsies and the crevicular fluid of patients with various degrees of periodontal inflammation [3,15,18,20-22]. Although normal tissues do not express MMPs and constitutive expression is in minimal, MMPs are transcriptionally regulated by growth factors, cytokines, and ECM components during inflammatory stimulation [23]. For example, MMPs are capable of degradation of the ECM and the degradation is induced by proinflammatory cytokines such as IL-1β and TNF-α, suggesting that the enzymes play a crucial role in the pathogenesis of periodontitis [15]. Therefore, the pathogenic mechanisms of periodontitis that are mediated by IL-1, IL-6, TNF-α, and MMPs are linked to each other [24,25]. For example, the production of MMPs during the acute phase of periodontitis is elevated by IL-1 or IL-6 produced by neutrophils [24].
In this study, the investigators observed that MMP-9, corresponding to molecular weight 94 kDa, was highly expressed in the gingival tissues of 13 periodontal patients, while MMP-2, corresponding to molecular weight 72 kDa, was underexpressed compared with MMP-9 (Fig. 1). It has been suggested that MMP-9 levels in periodontitis and gingivitis patients were higher than those in healthy subjects [17,26]. However, crevicular MMP-2 showed little expression in gingivitis and periodontitis, with expression levels lower than in a healthy group [26]. The present results obtained from the Western blot analysis for MMP-9 and -2 are in agreement with previous findings. Moreover, the detection of gelatin activity by gelatin zymography indicated that MMP-9 plays a more central role than MMP-2 (Fig. 2). In the present study, the expression and activity of MMP-9 and -2 are different in a given patient. It seems that patients have different correlations between the expression and activity of MMP-9 and -2. As shown in Fig. 3, the correlation between the expression and the activity of MMP-9 in all gingival tissue samples was significant (r=0.793, P=0.01). However, the study found no significant relationship between the expression and the activity of MMP-2. From these results, it is suggested that MMP-2 and -9 may be regulated by different mechanisms in periodontitis. Consequently, further studies will be required to elucidate this mechanism, because MMP activation and activity can be controlled by inhibition in several ways that include proteolytic degradation and inactivation, nonspecific endogenous inhibitors such as a 2-macroglobulin, and especially by specific tissue inhibitors of MMPs, tissue inhibitors of metalloproteinases [12,27].
In summary, Western blot analysis and a gelatin zymography assay revealed that the expression and activity of MMP-9 were higher than MMP-2 in 13 human subjects with periodontitis. Notably, MMP-9 expression was positively correlated with its activity, whereas for MMP-2 there was no correlation. The study suggested that the degree of MMP-9 expression and activity are more predictive indicators for periodontitis than MMP-2.
Figures and Tables
Figure 1
Expression of matrix metalloproteinase (MMP)-9 and -2 in patients with periodontitis. To analyze the expression of MMP-9 and -2, 100 µg of protein extracted from each of 13 samples was analyzed by Western blot. MMP-9, corresponding to molecular weight 94 kDa, and MMP-2, corresponding to molecular weight 72 kDa, were shown to be expressed in all of the samples. The numbers '1 to 13' indicate the number of each patient.
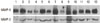
Figure 2
Matrix metalloproteinase (MMP)-2 and -9 gelatinolytic activity in patients with periodontitis. To analyze the activity of MMP-9 and -2, 10 µg of protein extracted from each of 13 samples was analyzed by gelatin zymography. Bands corresponding to MMP-9 and -2 can be observed in all samples. The numbers '1 to 13' indicate the number of each patient.

Figure 3
The correlation between the activity and expression of matrix metalloproteinase (MMP)-9 and -2 in patients with periodontitis. (A) Comparison of expression and activity of MMP-9 (upper panel) and -2 (lower panel) in individual patients. (B) Correlation between the activity and expression of MMP-9 and -2 in patients with periodontitis.

Table 1
Demographic and laboratory data of the 13 patients with periodontitis.

Expression and activity of MMP-9 and -2 is shown by an arbitrary unit (average of three different experiments). Each arbitrary unit was determined by densitometry analysis of the scanned images. All proteins were normalized per protein, 100 µg (Western) or 10 µg (zymography) protein per sample.
MMP: matrix metalloproteinase.
ACKNOWLEDGEMENTS
This study was supported by the Bio R&D program through the Korea National Research Foundation funded by the Ministry of Education, Science and Technology (Grant No. 2009-0092562).
References
1. Van Dyke TE, Serhan CN. Resolution of inflammation: a new paradigm for the pathogenesis of periodontal diseases. J Dent Res. 2003. 82:82–90.

2. DeCarlo AA Jr, Windsor LJ, Bodden MK, Harber GJ, Birkedal-Hansen B, Birkedal-Hansen H. Activation and novel processing of matrix metalloproteinases by a thiol-proteinase from the oral anaerobe Porphyromonas gingivalis. J Dent Res. 1997. 76:1260–1270.

3. Pozo P, Valenzuela MA, Melej C, Zaldivar M, Puente J, Martinez B, et al. Longitudinal analysis of metalloproteinases, tissue inhibitors of metalloproteinases and clinical parameters in gingival crevicular fluid from periodontitis-affected patients. J Periodontal Res. 2005. 40:199–207.

4. Vernal R, Dezerega A, Dutzan N, Chaparro A, Leon R, Chandia S, et al. RANKL in human periapical granuloma: possible involvement in periapical bone destruction. Oral Dis. 2006. 12:283–289.

5. Sorsa T, Tjaderhane L, Konttinen YT, Lauhio A, Salo T, Lee HM, et al. Matrix metalloproteinases: contribution to pathogenesis, diagnosis and treatment of periodontal inflammation. Ann Med. 2006. 38:306–321.

6. Birkedal-Hansen H. Role of cytokines and inflammatory mediators in tissue destruction. J Periodontal Res. 1993. 28(6 Pt 2):500–510.

7. Cazalis J, Tanabe S, Gagnon G, Sorsa T, Grenier D. Tetracyclines and chemically modified tetracycline-3 (CMT-3) modulate cytokine secretion by lipopolysaccharide-stimulated whole blood. Inflammation. 2009. 32:130–137.

8. Uitto VJ, Overall CM, McCulloch C. Proteolytic host cell enzymes in gingival crevice fluid. Periodontol 2000. 2003. 31:77–104.

9. Sorsa T, Tervahartiala T, Leppilahti J, Hernandez M, Gamonal J, Tuomainen AM, et al. Collagenase-2 (MMP-8) as a point-of-care biomarker in periodontitis and cardiovascular diseases. Therapeutic response to non-antimicrobial properties of tetracyclines. Pharmacol Res. 2011. 63:108–113.

10. Cox SW, Eley BM, Kiili M, Asikainen A, Tervahartiala T, Sorsa T. Collagen degradation by interleukin-1beta-stimulated gingival fibroblasts is accompanied by release and activation of multiple matrix metalloproteinases and cysteine proteinases. Oral Dis. 2006. 12:34–40.

11. Reynolds JJ, Meikle MC. Mechanisms of connective tissue matrix destruction in periodontitis. Periodontol 2000. 1997. 14:144–157.

12. Ryan ME, Golub LM. Modulation of matrix metalloproteinase activities in periodontitis as a treatment strategy. Periodontol 2000. 2000. 24:226–238.

13. Sorsa T, Tjaderhane L, Salo T. Matrix metalloproteinases (MMPs) in oral diseases. Oral Dis. 2004. 10:311–318.

14. Ingman T, Sorsa T, Michaelis J, Konttinen YT. Matrix metalloproteinases-1, -3, and -8 in adult periodontitis in situ. An immunohistochemical study. Ann N Y Acad Sci. 1994. 732:459–461.
15. Corotti MV, Zambuzzi WF, Paiva KB, Menezes R, Pinto LC, Lara VS, et al. Immunolocalization of matrix metalloproteinases-2 and -9 during apical periodontitis development. Arch Oral Biol. 2009. 54:764–771.

16. Tsagareli ZG, Shishniashvili TE, Gogiashvili LE, Kvachadze TI, Khimshiashvili NB. The level of matrix metalloproteinases and type IV collagen in the gingival mucosa under different clinical forms of periodontitis in pre- and pubertal periods and their prognostic value. Georgian Med News. 2012. (206):25–29.
17. Rai B, Kharb S, Jain R, Anand SC. Biomarkers of periodontitis in oral fluids. J Oral Sci. 2008. 50:53–56.

18. Nishikawa M, Yamaguchi Y, Yoshitake K, Saeki Y. Effects of TNFalpha and prostaglandin E2 on the expression of MMPs in human periodontal ligament fibroblasts. J Periodontal Res. 2002. 37:167–176.

19. Reynolds JJ, Hembry RM, Meikle MC. Connective tissue degradation in health and periodontal disease and the roles of matrix metalloproteinases and their natural inhibitors. Adv Dent Res. 1994. 8:312–319.

20. Emingil G, Kuula H, Sorsa T, Atilla G. Gingival crevicular fluid matrix metalloproteinase-25 and -26 levels in periodontal disease. J Periodontol. 2006. 77:664–671.

21. Tervahartiala T, Pirila E, Ceponis A, Maisi P, Salo T, Tuter G, et al. The in vivo expression of the collagenolytic matrix metalloproteinases (MMP-2, -8, -13, and -14) and matrilysin (MMP-7) in adult and localized juvenile periodontitis. J Dent Res. 2000. 79:1969–1977.

22. Aiba T, Akeno N, Kawane T, Okamoto H, Horiuchi N. Matrix metalloproteinases-1 and -8 and TIMP-1 mRNA levels in normal and diseased human gingivae. Eur J Oral Sci. 1996. 104:562–569.

23. Shapiro SD, Senior RM. Matrix metalloproteinases. Matrix degradation and more. Am J Respir Cell Mol Biol. 1999. 20:1100–1102.
24. Márton IJ, Kiss C. Protective and destructive immune reactions in apical periodontitis. Oral Microbiol Immunol. 2000. 15:139–150.

25. Kesanakurti D, Chetty C, Bhoopathi P, Lakka SS, Gorantla B, Tsung AJ, et al. Suppression of MMP-2 attenuates TNF-α induced NF-κB activation and leads to JNK mediated cell death in glioma. PLoS One. 2011. 6:e19341.

26. Maeso G, Bravo M, Bascones A. Levels of metalloproteinase-2 and -9 and tissue inhibitor of matrix metalloproteinase-1 in gingival crevicular fluid of patients with periodontitis, gingivitis, and healthy gingiva. Quintessence Int. 2007. 38:247–252.
27. Murphy G, Docherty AJ, Hembry RM, Reynolds JJ. Metalloproteinases and tissue damage. Br J Rheumatol. 1991. 30:Suppl 1. 25–31.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download