Abstract
Purpose
The level of the implant above the marginal bone and flap design have an effect on the bone resorption during the healing period. The aim of this study is to detect the relationship between the level of the implant at the implant placement and the bone level at the healing period in the mesial and distal side of implants placed with flapless (FL) and full-thickness flap (FT) methods.
Methods
Twenty-two nonsubmerged implants were placed with the FL and FT technique. Periapical radiographs were taken of the patient at implant placement, and at 6 and 12 weeks. By using computer software, bone level measurements were taken from the shoulder of the healing cap to the first bone implant contact in the mesial and distal side of the implant surface.
Results
At 6 weeks, the correlation between the crestal bone level at the implant placement and crestal bone level of the FT mesially was significant (Pearson correlation coefficient=0.675, P<0.023). At 12 weeks, in the FT mesially, the correlation was nonsignificant (Spearman correlation coefficient=0.297, P<0.346). At 6 weeks in the FT distally, the correlation was nonsignificant (Pearson correlation coefficient=0.512, P<0.107). At 12 weeks in the FT distally, the correlation was significant (Spearman correlation coefficient=0.730, P<0.011). At 6 weeks in the FL mesially, the correlation was nonsignificant (Spearman correlation coefficient=0.083, P<0.809). At 12 weeks in the FL mesially, the correlation was nonsignificant (Spearman correlation coefficient=0.062, P<0.856). At 6 weeks in the FL distally, the correlation was nonsignificant (Spearman correlation coefficient=0.197, P<0.562). At 12 weeks in the FL distally, the correlation was significant (Pearson correlation coefficient=0.692, P<0.018).
Crestal bone resorption around an implant begins at the time of implant placement and proceeds through the loading period and the period after loading the implant. The factors that affect crestal bone may vary and can include the implant design, surgical technique, biological width, prosthetic design, and loading factor. The nonsubmerged implant with a smooth collar above the marginal bone is considered a subject of interest. As previous studies have explained, the cause of bone resorption in this type of implant is due to over-sinking of the implant inside the socket, causing a smooth surface to face the bone. When the smooth surface comes into contact with bone, shear stress will be transmitted to the crestal bone [1]. Alveolar bone is more tolerant of compressive forces, while it is less resistant to shear stress, and this will enhance bone resorption [2-7].
Others believe the cause of bone resorption in nonsubmerged implants is due to the biological width formation for the attachment of epithelial and connective tissue [8]. One of the advantages of nonsubmerged implants is the formation of biological width during the early healing period [9]. From a previous study [10], it was determined that biological width formation requires a 3-mm depth for the sulcus depth, junctional epithelium, and connective tissue seal. Therefore, the biological width needs space to be formed, and this may also enhance bone resorption around the implant. Another factor is that a nonsubmerged dental implant is exposed to the oral cavity and it is not covered by the gingival tissue during the healing period as in a submerged implant [11,12]. For this, the implant has a greater chance to be involved with contamination with the saliva and plaque accumulation that contain bacteria. If a chronic irritant, such as bacteria, reaches the crestal bone around the implant, bone resorption occurs, creating a separation from the irritated area [9,13,14]. Among the factors that affect crestal bone resorption is periosteum reflection when the full-thickness flap (FT) is raised. An implant placed with the flapless (FL) technique is thought to preserve the blood vessels in the inner layer of the periosteum from cutting and as a consequence, prevent the bone from resorption [15,16].
The FL technique has now become a more predictable method, not only because of periosteum and crestal bone preservation but also because of less pain, less time consumed, the decrease in the bleeding during the operation and less morbidity postoperatively [17-23].
The aim of the present study is to evaluate the influence of the implant level at implant placement on the bone level in the FT and FL techniques during the healing period.
In an experimental prospective clinical study, each patient will receive 2 implants in the posterior of the maxilla or mandible. The patients will be followed at 6 weeks and 12 weeks after implant placement to collect data.
This study was approved by the Research and Ethics Committee at the School of Medical Sciences Universiti Sains Malaysia, Kubang Kerian, Kelantan USMKK/PPP/JEPem/[200.3 (6)] on March 9, 2008.
All of the procedures to be performed were explained verbally and in writing to the patients, and all of the patients signed an informed consent form for the implant placement surgical procedure.
The inclusion criteria included patients above 18 years of age with the posterior mandibular and maxillary missing teeth. The exclusion criteria were being unfit for a surgical procedure, immediate implant, and needing alveolar bone grafting or maxillary sinus augmentation.
After administration of local anesthesia by mepivacaine hydrochloride (2%) with adrenalin 1:100,000, Scandonest (Septodont, Saint-Maur-des-fossés Cedex, France), a midcrestal incision was performed.
After mucoperiosteal flap reflection, the implant bed was prepared according to implant system recommendations. The appropriate implant position was selected and marked with a small round bur that could penetrate the outer cortex. The preparation of the implant bed was carried out with spiral drills of increasing diameter with copious normal saline irrigation, and an intermittent drilling technique
Bone taping was done for the type I and some of the type II bone. ITI sand-blasted, large grit, acid-etched (SLA) implants (Institut Straumann AG, Basel, Switzerland) with a regular neck, length of 10 mm, and a diameter of either 4.1 mm or 4.8 mm were used.
The implant was placed manually into its final position with the aid of a ratchet. The nonsubmerged implant was left at 2.8 mm above the crestal bone, the insertion torque was recorded, and it ranged from 20 to 35 Ncm. Only one implant in the maxillary molar area was recorded to be below 20 Ncm. A healing cap was placed, and the flap was repositioned, adapted, and sutured.
The FL technique was done by a manual disposable biopsy tissue punch (Nobel Biocare, Goteborg, Sweden), which was used to make a circular excision through the soft tissue and periosteum. Implant bed preparation was performed in the same was as in the FT technique according to the implant system recommendation and the healing cap was screwed on.
A periapical radiograph was taken by the Oralix AC system (Genedex dental system, Milan, Italy). The periapical film used was a photosensitive phosphor plate Gendex size 3 (Genedex dental system). Using the long-cone parallel technique, special care was taken to position these periapical films parallel to the implant. A Rinn film holder (Dentsply International Inc., York, PA, USA) was used to align the radiographic beam perpendicular to the implant, and served as the baseline for crestal bone level determination (Fig. 1). Since the implant in this study was a threaded implant, when the threads were not visible in the radiograph and there was overlapping, it meant that the film and the implant were not in a parallel position. These radiographs were repeated and were not included in this study. Periapical radiographs were taken at implant placement, and at 6 and 12 weeks afterward.
The periapical radiographs were evaluated after scanning by a scanner (Den Optix, Genedex dental system). The image was magnified with computer software (VixWin 2000, Genedex dental system).
The known value of the inter-thread distance for the implants (the distance between 2 threads was 1.25 mm) was used as an internal reference distance. By using the 'measurement tools' in the imaging software, linear measurements were taken mesially and distally from the shoulder of the healing cap (as a reference point) to the first bone-to-implant contact. An increase in the distance meant an increase in the crestal bone resorption, and a decrease in this distance meant there was bone gain.
The software used for the analysis of the data was SPSS ver. 12 (SPSS Inc., Chicago, IL, USA). The mean values of the measurement scores were calculated mesially and distally in the FL and FT group. The data were grouped using the implant surfaces as units for analysis. When the data were normally distributed in one of the correlated groups, Pearson correlation coefficients were used to determine the relationship between the crestal bone level at the implant placement and at 6- and 12-week intervals in the mesial and distal sides of the FL and FT groups. When the data were abnormally distributed in both groups, the Spearman correlation test was used. A P-value of <0.05 was considered significant.
The mean±standard deviation of the mesial/distal bone level of the FT and FL groups at implant placement, and 6 and 12 weeks is shown in Table 1. The correlation between the mesial crestal bone level of the FT at implant placement and mesial crestal bone level of the FT at 6 weeks was significant (P<0.023). The observed correlation coefficient (r) was 0.675, which suggests a positive good correlation (Pearson test, P<0.05) (Fig. 2).
The correlation between the mesial crestal bone level of the FT at implant placement and mesial crestal bone level of the FT at 12 weeks was not significant (P<0.346). The observed correlation coefficient (r) was 0.297, which suggested a positive fair correlation (Spearman test, P<0.05) (Fig. 3).
The correlation between the distal crestal bone level of the FT at implant placement and the distal crestal bone level of the FT at 6 weeks was not significant (P<0.107). The observed correlation coefficient (r) was 0.512, which suggested a positive good correlation (Pearson test, P<0.05) (Fig. 4).
The correlation between the distal crestal bone level FT at implant placement and distal crestal bone level at 12 weeks was significant (P<0.011). The observed correlation coefficient (r) was 0.730, which suggested a significant positive correlation (good correlation) (Spearman test, P<0.05) (Fig. 5).
The correlation between the mesial crestal bone level of the FL at implant placement and mesial crestal bone level at 6 weeks was not significant (P<0.809). The observed correlation coefficient (r) was 0.083, which suggested a positive poor correlation (Spearman test, P<0.05) (Fig. 6).
The correlation between the mesial crestal bone level of the FL at implant placement and mesial crestal bone level at 12 weeks was not significant (P<0.856). The observed correlation coefficient (r) was 0.062, which suggested a poor positive correlation (Spearman test, P<0.05) (Fig. 7).
The correlation between the distal crestal bone level of the FL at implant placement and distal crestal bone level at 6 weeks was not significant (P<0.562). The observed correlation coefficient (r) was 0.197, which suggests a positive poor correlation (Spearman test, P<0.05) (Fig. 8).
The correlation between the distal crestal bone level of the FL at implant placement and distal crestal bone level at 12 weeks was significant (P<0.018). The observed correlation coefficient (r) was 0.692, which suggested a positive good correlation (Pearson test, P<0.05) (Fig. 9).
The correlations between the implant level at implant placement and the bone level at 6 and 12 weeks later at the mesial and distal sides for the FT group were significant, except at the mesial side at 12 weeks, which was not significant. This indicates that less bone resorption occurs when the implant is placed deep into the prepared socket. In other words, the higher the implant is placed above the bone, the more bone resorption occurs. This is in accordance with the study of Todescan et al. [24] study, in which the authors placed 3 implants at different levels: above, with, and below the marginal bone. The authors found that the countersink group had less bone loss than the other groups. The implant that was used in this study was ITI SLA with a 2.8 mm smooth collar above the marginal bone and was placed as a nonsubmerged implant. While previous studies [25] using the same type of implant showed that when the smooth/rough border was placed under the crestal bone, bone resorption would be increased. In addition, the results of the present study are opposed to the conclusion that bone resorption around the nonsubmerged implant starts after implant placement to create space for the biological width to be established [26]. Since biological width formation requires a 3-mm depth: 0.49 mm for the sulcus depth, 1.16 mm for the junctional epithelium, and 1.36 mm for the connective tissue seal [10]. Therefore, the biological width needs space to form, and the implant that is placed deeper should have more bone resorption than the implant that is placed at a high level. The FL group had no significant correlation to the level of the implant at the implant placement. This indicates that the bone level in the FL group was not affected by the level of implant placement above the bone. The finding of the present study revealed that the FT placed implant showed more bone resorption when the implant was placed away from the marginal bone, while this relationship had not been found in the FL placed implant. The explanation of these findings is that when the implant was placed with FT and away from the marginal bone, this positioned the implant closer to the oral environment and plaque accumulation. In addition, with the difficulty of flap adaptation on the surface of the implant and suture loosening, the implant surface is more susceptible to exposure. All this allows the marginal bone to be invaded by the bacteria and harmed by the inflammation process and consequently causes bone resorption [9].
In addition to that, in the FT technique, the periosteum is disrupted and the blood vessels that supply the alveolar bone have been cut. Together, these conditions decrease the blood supply to the marginal bone, and consequently, the defense mechanism against bacteria also decreases.
The soft tissue barrier that surrounds the implant is composed of an epithelial component continuous with a zone of connective tissue [9]. The connective tissue in close contact with the surface of the implant is rich in collagen but poor in cells and vascular structures, resembling scar tissue [9]. Knowing that scar tissue contains less blood supply than normal tissue [24], this also allows the marginal bone around the implant to be easily invaded and destroyed by bacteria. The previous conclusion can also be supported by another finding of the current study regarding the FL group: It is not affected by the length of the implant above the marginal bone. This may be due to the rich blood supply to the alveolar bone when the periosteum and blood vessels were preserved from cutting in the FL group. This rich blood supply should provide a good defense mechanism against bacterial invasion. Another interesting finding in this study was that the distal side was highly correlated with crestal bone resorption than the mesial side. This can be seen in the distal side of the FL technique at 12 weeks, where the correlation was significant, while the mesial side of the FT at 12 weeks was not significant. This is in accordance with previous studies [1,4,27-29] found that the distal side has more bone resorption than the mesial side. A large sample size is recommended to verify the conclusions in this preliminary study.
In conclusion, within the limitations of this study, the initial implant position in FT group has greater influence on the crestal bone resorption and was positively correlated with it. Furthermore, the distal side of the FT and FL placed implant groups was more positively correlated with crestal bone resorption than the mesial side.
Figures and Tables
 | Figure 1Using the long-cone parallel technique with a Rinn film holder (Dentsply International Inc.). |
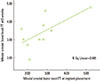 | Figure 2Correlation between the mesial crestal bone level of the full-thickness flap (FT) at implant placement and the mesial crestal bone level of the FT at 6 weeks (mm). There was a significant positive correlation (moderate to good correlation) (r=0.675, P<0.023). |
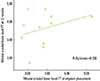 | Figure 3Correlation between the mesial crestal bone level of the full-thickness flap (FT) at implant placement and the mesial crestal bone level of the FT at 12 weeks (mm). There was a fair statistically nonsignificant positive correlation (r=0. 297, P<0.346). |
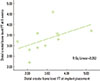 | Figure 4Correlation between the distal crestal bone level of the full-thickness flap (FT) at implant placement and the distal crestal bone level of the FT at 6 weeks (mm). There was a nonsignificant positive correlation (moderate correlation) (r=0. 512, P<0.107). |
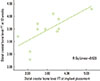 | Figure 5Correlation between the distal crestal bone level of the full-thickness flap (FT) at implant placement and the distal crestal bone level of the FT group at 12 weeks (mm). There was a significant positive correlation (good correlation) (r=0.730, P<0.011). |
 | Figure 6Correlation between the mesial crestal bone level of the flapless (FL) at implant placement and the mesial crestal bone level of the FL at 6 weeks (mm). There was a nonsignificant positive correlation (poor correlation) (r=0.083, P<0.809). |
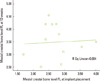 | Figure 7Correlation between the mesial crestal bone level of the flapless (FL) at implant placement and the mesial crestal bone level of the FL at 12 weeks (mm). There was a non-significant positive correlation (poor correlation) (r=0.062, P<0.856). |
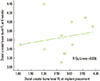 | Figure 8Correlation between the distal crestal bone level of the flapless (FL) at implant placement and the distal crestal bone level of the FL at 6 weeks (mm). There was a poor nonsignificant positive correlation (r=0.197, P<0.562). |
References
1. Bergkvist G, Sahlholm S, Nilner K, Lindh C. Implant-supported fixed prostheses in the edentulous maxilla. A 2-year clinical and radiological follow-up of treatment with non-submerged ITI implants. Clin Oral Implants Res. 2004; 15:351–359.

2. Bischof M, Nedir R, Abi Najm S, Szmukler-Moncler S, Samson J. A five-year life-table analysis on wide neck ITI implants with prosthetic evaluation and radiographic analysis: results from a private practice. Clin Oral Implants Res. 2006; 17:512–520.

3. Soadoun AP, Touati B. Soft tissue recession around implants: is it still unavoidable?--Part II. Pract Proced Aesthet Dent. 2007; 19:81–87.
4. Brägger U, Hafeli U, Huber B, Hammerle CH, Lang NP. Evaluation of postsurgical crestal bone levels adjacent to non-submerged dental implants. Clin Oral Implants Res. 1998; 9:218–224.

5. Oh TJ, Shotwell JL, Billy EJ, Wang HL. Effect of flapless implant surgery on soft tissue profile: a randomized controlled clinical trial. J Periodontol. 2006; 77:874–882.

6. Ostman PO, Hellman M, Albrektsson T, Sennerby L. Direct loading of Nobel Direct and Nobel Perfect one-piece implants: a 1-year prospective clinical and radiographic study. Clin Oral Implants Res. 2007; 18:409–418.

7. Lee DW, Choi YS, Park KH, Kim CS, Moon IS. Effect of microthread on the maintenance of marginal bone level: a 3-year prospective study. Clin Oral Implants Res. 2007; 18:465–470.

8. Hartman GA, Cochran DL. Initial implant position determines the magnitude of crestal bone remodeling. J Periodontol. 2004; 75:572–577.

9. Novaes AB Jr, de Oliveira RR, Muglia VA, Papalexiou V, Taba M. The effects of interimplant distances on papilla formation and crestal resorption in implants with a morse cone connection and a platform switch: a histomorphometric study in dogs. J Periodontol. 2006; 77:1839–1849.

10. Cochran DL, Hermann JS, Schenk RK, Higginbottom FL, Buser D. Biologic width around titanium implants: a histometric analysis of the implanto-gingival junction around unloaded and loaded nonsubmerged implants in the canine mandible. J Periodontol. 1997; 68:186–198.

11. Hürzeler M, Fickl S, Zuhr O, Wachtel HC. Peri-implant bone level around implants with platform-switched abutments: preliminary data from a prospective study. J Oral Maxillofac Surg. 2007; 65:7 Suppl 1. 33–39.

12. Choi BH, Li J, Kim HS, Ko CY, Jeong SM, Xuan F. Comparison of submerged and nonsubmerged implants placed without flap reflection in the canine mandible. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008; 105:561–565.

13. Machtei EE, Oved-Peleg E, Peled M. Comparison of clinical, radiographic and immunological parameters of teeth and different dental implant platforms. Clin Oral Implants Res. 2006; 17:658–665.

14. Papalexiou V, Novaes AB Jr, Ribeiro RF, Muglia V, Oliveira RR. Influence of the interimplant distance on crestal bone resorption and bone density: a histomorphometric study in dogs. J Periodontol. 2006; 77:614–621.

15. Jeong SM, Choi BH, Li J, Kim HS, Ko CY, Jung JH, et al. Flapless implant surgery: an experimental study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007; 104:24–28.

16. Gomez-Roman G. Influence of flap design on peri-implant interproximal crestal bone loss around single-tooth implants. Int J Oral Maxillofac Implants. 2001; 16:61–67.
17. Ozan O, Turkyilmaz I, Yilmaz B. A preliminary report of patients treated with early loaded implants using computerized tomography-guided surgical stents: flapless versus conventional flapped surgery. J Oral Rehabil. 2007; 34:835–840.

18. al-Ansari BH, Morris RR. Placement of dental implants without flap surgery: a clinical report. Int J Oral Maxillofac Implants. 1998; 13:861–865.
19. Auty C, Siddiqui A. Punch technique for preservation of interdental papillae at nonsubmerged implant placement. Implant Dent. 1999; 8:160–166.

21. Marchack CB. CAD/CAM-guided implant surgery and fabrication of an immediately loaded prosthesis for a partially edentulous patient. J Prosthet Dent. 2007; 97:389–394.

22. Al-Juboori MJ, Bin Abdulrahaman S, Jassan A. Comparison of flapless and conventional flap and the effect on crestal bone resorption during a 12-week healing period. Dent Implantol Update. 2012; 23:9–16.
23. Al-Juboori MJ, bin Abdulrahaman S, Subramaniam R, Tawfiq OF. Less morbidity with flapless implant. Dent Implantol Update. 2012; 23:25–30.
24. Todescan FF, Pustiglioni FE, Imbronito AV, Albrektsson T, Gioso M. Influence of the microgap in the peri-implant hard and soft tissues: a histomorphometric study in dogs. Int J Oral Maxillofac Implants. 2002; 17:467–472.
25. Hermann JS, Schoolfield JD, Nummikoski PV, Buser D, Schenk RK, Cochran DL. Crestal bone changes around titanium implants: a methodologic study comparing linear radiographic with histometric measurements. Int J Oral Maxillofac Implants. 2001; 16:475–485.
26. Hermann JS, Buser D, Schenk RK, Higginbottom FL, Cochran DL. Biologic width around titanium implants. A physiologically formed and stable dimension over time. Clin Oral Implants Res. 2000; 11:1–11.

27. Tarnow DP, Cho SC, Wallace SS. The effect of inter-implant distance on the height of inter-implant bone crest. J Periodontol. 2000; 71:546–549.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download