Abstract
Purpose
An animal periodontitis model is essential for research on the pathogenesis and treatment of periodontal disease. In this study, we have introduced a lipopolysaccharide (LPS) of a periodontal pathogen to the alveolar bone defect of experimental animals and investigated its suitability as a periodontitis model.
Methods
Alveolar bone defects were made in both sides of the mandibular third premolar region of nine beagle dogs. Then, the animals were divided into the following groups: silk ligature tied on the cervical region of tooth group, Porphyromonas gingivalis LPS (P.g. LPS)-saturated collagen with silk ligature group, and no ligature or P.g. LPS application group as the control. The plaque index and gingival index were measured at 0 and 4 weeks postoperatively. The animals were then euthanized and prepared for histologic evaluation.
Results
The silk ligature group and P.g. LPS with silk ligature group showed a significantly higher plaque index at 4 weeks compared to the control (P<0.05). No significant difference was found in the plaque index between the silk ligature group and P.g. LPS with silk ligature group. The P.g. LPS with silk ligature group showed a significantly higher gingival index compared to the silk ligature group or the control at 4 weeks (P<0.05). Histologic examination presented increased inflammatory cell infiltration in the gingival tissue and alveolar bone of the P.g. LPS with silk ligature group.
Periodontitis is a prevalent inflammatory dental disease that causes the destruction of tooth-supporting structures and eventually results in the loss of the tooth [1]. To study the phenomenon of periodontal inflammation and the effect of periodontal treatment, several animal models have been adopted [2-4]. Among these animal models, dogs are one of the commonly chosen models for periodontal study because of their high occurrence rate of periodontitis and the same etiologic factors as humans [5]. However, the natural periodontitis of dogs produces inconsistent periodontal lesions with an uneven extent and localization of periodontal inflammation [6]. Therefore, an experimental periodontitis model has been proposed.
It was previously reported that a surgically made critical-sized class III furcation defect in the mandibular premolar of dogs is a reliable acute defect model for periodontal regeneration procedures [7]. The surgical bone resection method does not lead to inflammatory bone loss, therefore, this sometimes makes it difficult to determine whether this model is practically applicable to human periodontitis treatment. To reproduce the periodontitis situation, a chronic defect model can be obtained by placing silk or cotton ligatures around tooth cervical areas, which takes over 4 months [8]. A combined model in which surgical defect formation after chronic inflammation induction has been reported, but a several-month waiting period is also required to achieve chronic periodontal inflammation [9,10].
Porphyromonas gingivalis (P.g.), which resides in subgingival bacterial flora, has been reported to be the major pathogen in chronic periodontitis [11]. A previous study showed that lipopolysaccharide (LPS) of pathogenic bacteria application induced periodontal inflammation [12]. The purpose of this study was to evaluate the experimental periodontitis condition in surgically created alveolar bone defects of dogs by using P.g. LPS.
Nine 18- to 24-month-old adult male beagle dogs around 10 kg each were used as study subjects. The animals were acclimatized for 1 week prior to experimentation. The animals received dental prophylaxis for a healthy periodontal condition. This study followed the protocols approved by the Institutional Animal Care and Use Committee of Seoul National University.
Ten miligram of P.g. LPS (InvivoGen, San Diego, CA, USA) was dissolved in 1 mL of Dulbecco's phosphate-buffered saline (calcium/magnesium free, Gibco BRL, San Diego, CA, USA), and 20 µL of the solution was saturated into the collagen (ACE Surgical Supply Co., Brockton, MA, USA).
All of the surgical procedures were performed under general and local anesthesia induced by intravenous injection of atropine (0.04 mg/kg), intramuscular injection of 2% xylazine hydrochloride (Bayer Korea Ltd, Seoul, Korea), and ketamine hydrochloride (Yuhan, Seoul, Korea). Routine dental infiltration anesthesia with 2% lidocaine hydrochloride/epinephrine 1:100,000 (Kwangmyung Pharmaceutical, Seoul, Korea) was used at the surgical site. After mucoperiosteal flap elevation on the mandibular third premolars of each dog, alveolar bone defects 5 mm in height and 5 mm in width were created on the buccal side. Then, the animals were divided into the following groups: 3-0 silk ligature (Ailee, Seoul, Korea) group, P.g. LPS-saturated collagen with 3-0 silk ligature tied on the cervical region of tooth group, and no ligature or P.g. LPS application group. For postsurgical care, 20 mg/kg cefazolin sodium (Yuhan) was administered. Then, soft diet feeding was provided.
The animals were euthanized 4 weeks after surgery. The mandibular block specimens were rinsed in sterile saline and fixed in 10% formaldehyde solution at a volume 10 times that of the block section for 10 days. Paraffin blocks were made and sectioned in the mesio-distal vertical plane, with a thickness of 5 µm, at 80 µm intervals. Each section was stained with hematoxylin and eosin, then examined under a bright-field microscope (BX 51, Olympus Co., Tokyo, Japan).
Before the surgical procedure, there was no significant difference in the plaque index among any of the groups (Fig. 1). The plaque indices of the control group, silk ligature group, and P.g. LPS with silk ligature group were 0.125±0.069, 0.167±0.078, and 0.167±0.078, respectively. Although the plaque index of all of the groups had increased after 4 weeks, the control group showed a significantly lower value (0.625±0.101) compared to the silk ligature group (1.79±0.134) or P.g. LPS with silk ligature group (1.75±0.138) (P<0.05). There was no significant difference between the silk ligature group and P.g. LPS with silk ligature group at 4 weeks.
Fig. 2 shows that none of the baseline gingival index values of any of the groups were significantly different. The control group value was 0.167±0.078, while those of the silk ligature group and P.g. LPS with silk ligature group were 0.208±0.085 and 0.167±0.078, respectively. On the other hand, at 4 weeks after surgery, significant differences were revealed among each of the groups. The P.g. LPS with silk ligature group showed a significantly higher value (2.208±0.159) compared to the silk ligature group (1.708±0.095) or the control (0.792±0.159) (P<0.05). There was a significant difference between the silk ligature group and the control at 4 weeks (P<0.05).
Fig. 3 demonstrates the severe inflammatory cell infiltration in the periodontal tissue of the P.g. LPS with silk ligature group. A huge loss of attachment along the root surface and apically spread inflammatory cell infiltration were evident. The silk ligature tied group showed gingival inflammation, which was milder than that of the P.g. LPS with silk ligature group. The control group showed minimal attachment loss and less inflammatory cell infiltration.
The alveolar bone of the P.g. LPS with silk ligature tied group showed severe inflammatory cell presence, while the silk ligature tied group showed reduced inflammation. As shown in Fig. 4, signs of inflammation in the alveolar bone can hardly be seen in the control group.
Periodontitis is one of the most prevalent diseases in humans, so many studies have used experimental animals to investigate its pathogenesis [15-17]. Dog model is widely used for periodontal study because periodontal inflammation is common in dogs and its pathological characteristics are similar to those of periodontitis in humans [5]. Periodontitis of dogs arises naturally from gingivitis with the aging process, but the time of onset is unpredictable and the defects are inconsistent, which are considered to be the drawbacks of this model [6].
Therefore, ligature placement in the teeth of dogs has been proposed to obtain an experimental periodontitis condition more quickly than periodontitis naturally occurs [18]. This chronic defect model takes over 4 months, and mainly destroys the interproximal bone, which is not suitable for periodontal treatment research [8]. In order to investigate the periodontal healing capacity of a therapeutic modality, surgically created class III furcation defects in the mandibular premolar area were introduced [7]. This model provided critical-sized defects; however, they are acute defects, which are not regarded as periodontal inflammation. A combined model, a surgical defect with chronic inflammation, has also been proposed [10]. Despite the gingival inflammation that occurred in this model, the surgical defect was not directly influenced by experimental periodontitis. Herein, we modified the surgical bone defect to be influenced by periodontal inflammation for a new combined experimental periodontitis model.
It was previously reported that P.g. comprises a major portion of bacteria in the dental plaque of dogs, and its quantity increases with age, plaque amount, and gingival inflammation [19]. LPS comprises the outer surface of all subgingival gram-negative bacteria, and it can induce inflammation in periodontal tissue [20,21]. LPS injection induced periodontitis in a rat model, which has been regarded as a time-saving method for producing experimental periodontitis [12,22].
Because the gingival sulcus is absent in a periodontally healthy dog, a proper plaque accumulation method is required to produce gingival inflammation [23-25]. Ligature placement on the cervical area of the tooth can collect dental plaque, and plaque bacteria provokes gingival inflammation [26]. Because it takes several months to produce periodontitis in this chronic model, additional measures are needed to achieve periodontal inflammation within a short period of time.
In our study, the P.g. LPS-saturated collagen with silk ligature group had developed periodontal inflammation that affected the gingiva and alveolar bone at 4 weeks after surgical defect creation. Although the plaque index was similar to that of the silk ligature group, the gingival index was significantly higher and the alveolar bone was highly infiltrated by inflammatory cells in the P.g. LPS-saturated collagen with silk ligature group.
Our findings are consistent with the results of previous studies that bone tissue may be influenced by LPS through inflammatory cytokines [27,28]. It has been proposed that LPS can affect circulating leukocytes directly and activate osteoclasts [29]. Based on these findings, we can deduce that the P.g. LPS in our study plays an important role in alveolar bone defects and may prevent compensatory bone formation. In our model, P.g. LPS directly influenced periodontal tissue including alveolar bone, and accumulated plaque bacteria in the silk ligature also affected the gingival tissue.
It has not been reported that P.g. alone can colonize periodontal lesions in a dog model, which is possibly due to the variability of periodontitis development in a dog model [30]. In the present study, we utilized collagen material to be saturated with P.g. LPS and also added silk ligature placement. Plaque bacteria might have been absorbed in P.g. LPS-saturated collagen and might have aggravated the periodontal inflammation. However, more evidence is needed. Our study has some limitations, in that the sample size is small and a P.g. LPS-deficient collagen with silk ligature group was not adopted, requiring further research.
Within the limits of this study, we were able to achieve periodontal inflammation at 4 weeks after surgical bone defect creation by using P.g. LPS-saturated collagen with silk ligature. This could be a possible candidate for an experimental periodontitis model for surgically created alveolar bone defects.
Figures and Tables
Figure 1
Plaque index for each group. There were no significant differences in any of the groups at baseline (week 0). After 4 weeks, the silk ligature group (Ligature) and silk ligature with Porphyromonas gingivalis lipopolysaccharide (P.g. LPS) group (Ligature+LPS) showed marked differences compared to the control (P<0.05). There were no significant differences between the silk ligature with P.g. LPS group and silk ligature group. Different asterisks indicate statistically significant differences. Data are presented as mean±standard error.

Figure 2
Gingival index for each group. There were no significant differences among the groups at week 0. After 4 weeks, the silk ligature with Porphyromonas gingivalis lipopolysaccharide (LPS) group (Ligature+LPS) showed significant differences compared to the other groups (P<0.05). There were also significant differences between the silk ligature group (Ligature) and the control. Different asterisks indicate statistically significant differences. Data are presented as mean±standard error.

Figure 3
Histologic views of each group (H&E, bar=1 mm). (A) The control group showed minimal attachment loss and very little inflammation infiltration. (B) The silk ligature group showed mild gingival inflammation. (C) The Porphyromonas gingivalis lipopolysaccharide with silk ligature group showed severe inflammatory cell infiltration.
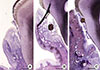
Figure 4
Alveolar bone images of each group (H&E, bar=100 µm). (A) There was no sign of inflammation in the control. (B) The silk ligature group showed reduced inflammation. (C) The Porphyromonas gingivalis lipopolysaccharide with silk ligature group showed a severe inflammatory cell presence in the alveolar bone tissue.

ACKNOWLEDGEMENTS
This study was supported by a grant of the Korean Health Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A101768).
References
2. Schou S, Holmstrup P, Kornman KS. Non-human primates used in studies of periodontal disease pathogenesis: a review of the literature. J Periodontol. 1993; 64:497–508.

3. Yamasaki A, Nikai H, Niitani K, Ijuhin N. Ultrastructure of the junctional epithelium of germfree rat gingiva. J Periodontol. 1979; 50:641–648.

4. Eggert FM, Germain JP, Cohen B. The gingival epithelium of rodent molars with limited eruption. Acta Anat (Basel). 1980; 107:297–306.

5. Attström R, Graf-de Beer M, Schroeder HE. Clinical and histologic characteristics of normal gingiva in dogs. J Periodontal Res. 1975; 10:115–127.

6. Haney JM, Zimmerman GJ, Wikesjo UM. Periodontal repair in dogs: evaluation of the natural disease model. J Clin Periodontol. 1995; 22:208–213.

7. Wikesjö UM, Kean CJ, Zimmerman GJ. Periodontal repair in dogs: supraalveolar defect models for evaluation of safety and efficacy of periodontal reconstructive therapy. J Periodontol. 1994; 65:1151–1157.

8. Holland M, Boring JG, Boyle CR, Pickrum HM, Jeffcoat MK. Radiographic bone loss correlations and technetium-99m-MDP bone uptake in ligature-induced periodontal disease in the beagle. Vet Radiol Ultrasound. 1998; 39:366–374.

9. Clergeau LP, Danan M, Clergeau-Guerithault S, Brion M. Healing response to anorganic bone implantation in periodontal intrabony defects in dogs. Part I. Bone regeneration. A microradiographic study. J Periodontol. 1996; 67:140–149.

10. Hayashi C, Kinoshita A, Oda S, Mizutani K, Shirakata Y, Ishikawa I. Injectable calcium phosphate bone cement provides favorable space and a scaffold for periodontal regeneration in dogs. J Periodontol. 2006; 77:940–946.

11. Andrian E, Grenier D, Rouabhia M. Porphyromonas gingivalis-epithelial cell interactions in periodontitis. J Dent Res. 2006; 85:392–403.

12. Dumitrescu AL, Abd-El-Aleem S, Morales-Aza B, Donaldson LF. A model of periodontitis in the rat: effect of lipopolysaccharide on bone resorption, osteoclast activity, and local peptidergic innervation. J Clin Periodontol. 2004; 31:596–603.

13. Silness J, Loe H. Periodontal disease in pregnancy: II. Correlation between oral hygiene and periodontal condtion. Acta Odontol Scand. 1964; 22:121–135.

14. Loe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand. 1963; 21:533–551.

15. Page RC, Schroeder HE. Pathogenesis of inflammatory periodontal disease: a summary of current work. Lab Invest. 1976; 34:235–249.
16. Lallam-Laroye C, Escartin Q, Zlowodzki AS, Barritault D, Caruelle JP, Baroukh B, et al. Periodontitis destructions are restored by synthetic glycosaminoglycan mimetic. J Biomed Mater Res A. 2006; 79:675–683.

17. Peruzzo DC, Benatti BB, Antunes IB, Andersen ML, Sallum EA, Casati MZ, et al. Chronic stress may modulate periodontal disease: a study in rats. J Periodontol. 2008; 79:697–704.

18. Lindhe J, Hamp S, Loe H. Experimental periodontitis in the beagle dog. J Periodontal Res. 1973; 8:1–10.

19. Allaker RP, de Rosayro R, Young KA, Hardie JM. Prevalence of Porphyromonas and Prevotella species in the dental plaque of dogs. Vet Rec. 1997; 140:147–148.

20. Garrison SW, Nichols FC. LPS-elicited secretory responses in monocytes: altered release of PGE2 but not IL-1 beta in patients with adult periodontitis. J Periodontal Res. 1989; 24:88–95.

21. Page RC. The role of inflammatory mediators in the pathogenesis of periodontal disease. J Periodontal Res. 1991; 26(3 Pt 2):230–242.

22. Rogers JE, Li F, Coatney DD, Rossa C, Bronson P, Krieder JM, et al. Actinobacillus actinomycetemcomitans lipopolysaccharide-mediated experimental bone loss model for aggressive periodontitis. J Periodontol. 2007; 78:550–558.

23. Egelberg J. Local effect of diet on plaque formation and development of gingivitis in dogs: 3. Effect of frequency of meals and tube feeding. Odontol Revy. 1965; 16:50–60.
24. Hamp SE, Lindhe J, Loe H. Experimental periodontitis in the beagle dog. J Periodontal Res. 1972; (10):13–14.

25. Lindhe J, Hamp SE, Loe H. Experimental periodontitis in the beagle dog. Int Dent J. 1973; 23:432–437.

26. Lindhe J, Ericsson I. Effect of ligature placement and dental plaque on periodontal tissue breakdown in the dog. J Periodontol. 1978; 49:343–350.

27. Lindemann RA, Economou JS, Rothermel H. Production of interleukin-1 and tumor necrosis factor by human peripheral monocytes activated by periodontal bacteria and extracted lipopolysaccharides. J Dent Res. 1988; 67:1131–1135.

28. Garrison SW, Holt SC, Nichols FC. Lipopolysaccharide-stimulated PGE2 release from human monocytes. Comparison of lipopolysaccharides prepared from suspected periodontal pathogens. J Periodontol. 1988; 59:684–687.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download