Abstract
Purpose
The aim of this study was to identify a role for endodontic intervention in enhancing the regenerative potential of the periodontal ligament when combined with periodontal treatment in seriously involved teeth with a secondary endodontic component.
Methods
Patients who exhibited radiolucency extending to the periapical region, abnormal electric pulp testing values, and deep probing depth derived from primary periodontal disease with secondary endodontic involvement were included. Intentional root canal treatment was applied to those teeth in which the apical lesions were presumed to communicate with those of the periodontal lesion of the teeth that remained vital. In all three selected cases, regenerative periodontal therapy incorporating either bone graft or guided tissue regeneration was instituted 3 months after the endodontic intervention.
Results
Remarkable enhancement in radiographic density was noticeable around the affected teeth as evidenced by changes in radiopacity. There was a significant reduction in the probing pocket depth and gain in the clinical attachment level. Chewing discomfort gradually disappeared from the commencement of the combined treatment.
The term "endo-perio" lesion has been proposed to describe the destructive lesions resulting from inflammatory products found in varying degrees in both the periodontium and the pulpal tissues [1]. Endo-perio combined lesions include pulpal disease with secondary periodontal involvement (primary pulpal lesion), periodontal disease with secondary endodontic involvement (primary periodontal lesion), and true combined lesions [2]. As teeth with primary pulpal disease with secondary periodontal involvement and true combined lesions have already lost their vitality, endodontic treatment would be the best treatment option [3]. In the case of periodontal disease with secondary endodontic involvement, some teeth demonstrate necrotic pulp whereas others still retain their vitality [2].
Deep periodontal intraosseous lesions with secondary endodontic involvement usually occur when the downward progression of a periodontal pocket reaches the periapical tissues [4]. Primary periodontal lesions with secondary endodontic involvement generally end up with a resultant pulpal necrosis as they progress apically, yet the pulp often remains vital [2]. The reason is that even when pathologic changes occur in the pulp tissue influenced by destructive periodontal disease, the pulp usually does not undergo the degenerative process as long as the main canal is not affected [5]. Furthermore, although the pulp may demonstrate histologic evidence of inflammation, the potential for maintaining pulpal vitality remains if the main blood supply is not compromised [6]. Therefore, the treatment modality of primary periodontal lesions with secondary involvement of an endodontic lesion has been controversial.
In 1980, Casullo [7] classified combined endo-perio lesions into five categories and proposed that periodontal treatment alone be applied to periodontal disease with secondary endodontic involvement based on its periodontal origin. Gold and Moskow [6] have observed that an alternative treatment using both surgery and antibiotics without any concomitant endodontic therapy resulted in successful outcomes. Extensive damage to the periapical tissues may be treated without devitalizing a tooth if the lesion is of periodontal origin and properly diagnosed [5]. There is consensus among these studies that primary periodontal lesions should be managed only with periodontal therapy and, because of its origin, endodontic therapy would be unnecessary.
However, Bender and Seltzer [8] found that, among a group consisting of 57 teeth with periodontal disease but without caries or fillings, 79% of teeth showed pathological changes within the pulp. Such an untreated endodontic infection may influence the outcome of periodontal wound healing, leading to a higher risk of attachment loss [9]. As a result, pulpal conditions can influence marginal healing and repair as long as the affected dentinal tubules remain patent [10]. Without initial endodontic treatment, the exposure of dentinal tubules by the removal of cementum by rigorous scaling and root planning during the course of periodontal treatment will allow bacterial invasion of the tubules. This would increase the likelihood of cumulative damage to the pulp. Therefore, nontoxic intracanal therapeutic medicaments may be necessary to eliminate the bacteria and to help encourage tissue repair [1]. In summary, initial proper endodontic management can prevent pulpal infection and facilitate regenerative potential.
In the present study, patients who exhibited radiolucency extending to the periapical region, abnormal electric pulp testing values, and deep probing depth derived from primary periodontal disease with secondary endodontic involvement were included. We propose endodontic treatment prior to periodontal regenerative treatment for those teeth with secondary endodontic involvement in which communication with an apical lesion is present although the teeth remain vital.
This study protocol was approved by the Pusan National University Dental Hospital Institutional Review Board (#PNUDH-2013-012).
A 57-year-old male who had a chief complaint of dull pain in the upper right first premolar visited the Department of Periodontology, Pusan National University Dental Hospital. There was no remarkable systemic disease. A wide radiolucent lesion on the upper right first premolar was evident with a probing depth of 9 mm only in the palatal surface. The electric pulp testing result demonstrated a positive value (+8). The tooth was diagnosed as a primary periodontal lesion with secondary endodontic involvement based on the positive electric testing value with a wide periapical radiolucent lesion and deep probing depth. Accordingly, an intentional endodontic treatment plan was proposed, followed by regenerative periodontal treatment. At 3 months following the commencement of the intentional endodontic treatment, the palatal probing depth remained deep and a wide radiolucent lesion was still evident due to the untreated primary periodontal lesion. Regenerative periodontal treatment was scheduled to address the intraosseous defect without concern as to whether pulpal infection by periodontal treatment would occur. Upon flap reflection, an anorganic bovine bone (BBP, Oscotec Inc., Seoul, Korea) graft was placed into the lesion following palatal root debridement. Follow-up was performed every 3 months for 1 year. A radiographic image taken 1 year postoperatively showed a significant decrease in radiolucency suggestive of remarkable enhancement of the bone density around the affected region (Figs. 1 and 2).
A 55-year-old female presented with swelling and pus discharge on the labial aspect of the lower left first molar. The tooth had degree 1 mobility and was modestly positive (+7) according to the electric pulp test. The probing pocket depth was 10 mm at the distal sites. Radiographic images showed a wide and deep radiolucent lesion around the distal root. The diagnostic impression was a primary periodontal lesion with secondary endodontic involvement judged by the positive electric testing value with the wide periapical radiolucent lesion and deep probing depth. The treatment plan consisted of initial endodontic treatment followed by regenerative periodontal treatment. The swelling and pus discharge subsided after the endodontic treatment and root planing; however, the deep pockets on the distal root area remained given that the primary periodontal disease had not yet been fully managed. Regenerative periodontal therapy was scheduled to fill in the remaining defects following the endodontic treatment. Open flap debridement with an anorganic bovine bone (BBP) graft was performed and the intraosseous lesion healed uneventfully. Nine months following the surgical intervention, the radiographic images showed significant bone filling around the distal aspect of the distal root. The optimal level of occlusal function was restored and the patient was satisfied with the final outcomes (Figs. 3 and 4).
A 33-year-old male visited our periodontal clinic with a chief complaint of persistent dull pain on the lower left second molar on chewing and biting. The clinical condition was characterized by deep pocket depths ranging from 9 to 12 mm on the midbuccal and distal area, without a noticeable mobility or suppuration upon gentle probing. The result of the electric pulp tests indicated that the second molar was vital (+7), and the radiographic image revealed a circumferential radiolucency surrounding the distal root apex. The diagnostic impression was a primary periodontal lesion with secondary endodontic involvement based on a positive electric testing value with a wide periapical radiolucent lesion and deep probing depth. Intentional endodontic treatment followed by regenerative periodontal treatment was planned. Three months after the endodontic treatment, the guided tissue regeneration was performed for the resolution of the primary periodontal intraosseous lesion. The guided tissue regeneration consisted of the placement of a nonresorbable (expanded polytetrafluoroethylene) barrier membrane (Goretex, W.L. Gore and Associates, Newark, DE, USA) with anorganic bovine bone (BBP) graft. The membrane was removed at 6 weeks postoperatively. The lesion healed uneventfully and a radiograph taken at more than 6 months following the regenerative surgery demonstrated remarkable osseous repair and an enhanced radiopacity (Figs. 5 and 6).
Pulpal and periodontal tissues communicate with each other via various pathways such as the vascular system, apical foramen, and lateral canals. These patent foramina and canals can be potential communication routes of endodontic-periodontal inflammation [11]. The similarity in the composition of cellular infiltrates also implies the connection between the pulp and periodontal tissues. Taken together, these findings can support the notion of cross-contamination between the pulp and the periodontal tissues [12].
Periodontal inflammation may initially elicit degenerative change in the pulp. However, partial necrosis of the pulp may render a positive pulp testing value despite the existence of a combined lesion, especially in a multirooted tooth [13]. This is because the pulp usually does not undergo degenerative changes unless the main canal is involved, even when pathologic changes occur in the pulp derived from periodontal inflammation [5].
Some authors have warned that untreated endodontic infection can influence periodontal healing, with a higher risk of attachment loss [9]. The success rate of the endo-perio combined lesion without a concomitant regenerative procedure has been reported to range from 27% to 37% [14]. This result demonstrates the notably low success rate and explains why regenerative periodontal surgery should be performed following endodontic treatment in combined endo-perio lesions.
Proper diagnosis and adoption of the bone graft technique or the guided tissue regeneration technique combined with osseous grafting followed by the removal of etiological factors will only ensure complete restoration of the health and function to a tooth with severe attachment loss resulting from a combined endo-perio lesion [15,16]. Britain et al. [17] showed that management of induced endo-perio lesions by bioabsorbable collagen membranes alone or in combination with anorganic bovine bone matrix resulted in enhanced amounts of bone and periodontal ligament and significant increases in the amount of new cementum when compared to open flap debridement alone.
Many authors have claimed autogenous bone grafting to be the graft of choice for regenerative procedures [18]. However, a significant amount of bone regeneration is achievable with a xenograft and it removes the limitations of harvesting a sufficient amount of autogenous bone.
In the present case report, intentional endodontic treatment was performed prior to regenerative periodontal treatment in teeth with a positive electric pulp testing value that seemed abnormally high. We concluded that degenerative and necrotic changes of the pulp that had progressed due to long-standing periodontal inflammation despite the positive pulp testing value may suggest that the pulp was not totally necrotic. Regenerative periodontal treatment was scheduled to address the remaining intraosseous defect only after osseous healing was induced sufficiently by initial endodontic treatment. Although the target teeth in the present case report were diagnosed as having primary periodontal lesions with secondary endodontic involvement, intentionally scheduling an initial endodontic treatment could help reduce pain and prevent undesirable secondary pulpal infection during regenerative periodontal surgery. Following an intentional endodontic intervention, anorganic bovine bone grafts or guided tissue regeneration with a nonresorbable barrier membrane could successfully resolve an extensive intraosseous defect extending to the periapical region. Within the limitations of the present observations, an intentional endodontic intervention is proposed as a potential approach for the sophisticated management of teeth suffering from serious attachment loss and alveolar bone destruction with concomitant secondary endodontic involvement.
Figures and Tables
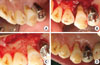 | Figure 1Initial intraoral clinical view (A) of upper right first premolar and at the time of periodontal surgery showing palatal bone defect and calculus deposit (B). Debridement and bone graft (xenograft) were performed (C). Clinical view at the time of 1-year recall check-up after endodontic and periodontal treatment (D). |
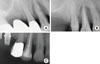 | Figure 2Periapical radiograph of upper right first premolar at initial visit (A) and at the time of 3-month recall check-up after endodontic treatment (B). There was no change in the radiographic bone defect morphology. Periapical radiograph at the time of 1-year recall check-up after endodontic and periodontal treatment (C). Note the changes of the radiolucency. |
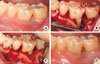 | Figure 3Initial intraoral clinical view of lower left first molar (A). Clinical view at the time of periodontal surgery showing distal circumferential bone defect (B). Debridement and bone graft (xenograft) were performed (C). Clinical view at the time of 9-month recall check-up after endodontic and periodontal treatment (D). |
 | Figure 4Periapical radiograph at the time of 3-month recall check-up after endodontic treatment (A). There was no change of bone defect in periapical radiograph compared to initial periapical radiograph (Unfortunately, initial radiograph is not shown here.). Periapical radiograph at the time of 9-month recall check-up after endodontic and periodontal treatment (B). |
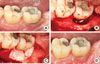 | Figure 5Initial intraoral clinical view of lower left second molar (A) and at the time of periodontal surgery showing distal circumferential bone defect (B). Debridement and bone graft (xenograft) and nonresorbable membrane (expanded polytetrafluoroethylene) adaptation were performed (C). Clinical view at the time of 1-year recall check-up after endodontic and periodontal treatment (D). |
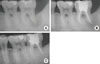 | Figure 6Initial periapical radiograph of lower left second molar showing circumferential bone defect around distal root (A). Periapical radiograph at the time of 3-month recall check-up after endodontic treatment (B). There was no change in the radiographic bone defect morphology. Periapical radiograph at the time of 1-year recall check-up after endodontic and periodontal treatment (C). |
ACKNOWLEDGEMENTS
This work was supported by Clinical Research Grant from Pusan National University Hospital (2011).
References
1. Singh P. Endo-perio dilemma: a brief review. Dent Res J (Isfahan). 2011; 8:39–47.
2. Simon JH, Glick DH, Frank AL. The relationship of endodontic-periodontic lesions. J Periodontol. 1972; 43:202–208.

3. Zehnder M, Gold SI, Hasselgren G. Pathologic interactions in pulpal and periodontal tissues. J Clin Periodontol. 2002; 29:663–671.

4. Rotstein I, Simon JH. Diagnosis, prognosis and decision-making in the treatment of combined periodontal-endodontic lesions. Periodontol 2000. 2004; 34:165–203.

5. Langeland K, Rodrigues H, Dowden W. Periodontal disease, bacteria, and pulpal histopathology. Oral Surg Oral Med Oral Pathol. 1974; 37:257–270.

6. Gold SI, Moskow BS. Periodontal repair of periapical lesions: the borderland between pulpal and periodontal disease. J Clin Periodontol. 1987; 14:251–256.

7. Casullo DP. The integration of endodontics, periodontics and restorative dentistry in general practice. Part I. Diagnosis. Compend Contin Educ Gen Dent. 1980; 1:137–147.
8. Bender IB, Seltzer S. The effect of periodontal disease on the pulp. Oral Surg Oral Med Oral Pathol. 1972; 33:458–474.

9. Jansson LE, Ehnevid H, Lindskog SF, Blomlof LB. Radiographic attachment in periodontitis-prone teeth with endodontic infection. J Periodontol. 1993; 64:947–953.

10. Blomlof L, Lindskog S, Hammarstrom L. Influence of pulpal treatments on cell and tissue reactions in the marginal periodontium. J Periodontol. 1988; 59:577–583.

11. Chen SY, Wang HL, Glickman GN. The influence of endodontic treatment upon periodontal wound healing. J Clin Periodontol. 1997; 24:449–456.

12. Bergenholtz G, Lekholm U, Liljenberg B, Lindhe J. Morphometric analysis of chronic inflammatory periapical lesions in root-filled teeth. Oral Surg Oral Med Oral Pathol. 1983; 55:295–301.

14. Oh SL, Fouad AF, Park SH. Treatment strategy for guided tissue regeneration in combined endodontic-periodontal lesions: case report and review. J Endod. 2009; 35:1331–1336.

15. Tseng CC, Harn WM, Chen YH, Huang CC, Yuan K, Huang PH. A new approach to the treatment of true-combined endodontic-periodontic lesions by the guided tissue regeneration technique. J Endod. 1996; 22:693–696.

16. Rankow HJ, Krasner PR. Endodontic applications of guided tissue regeneration in endodontic surgery. J Endod. 1996; 22:34–43.

17. Britain SK, Arx Tv, Schenk RK, Buser D, Nummikoski P, Cochran DL. The use of guided tissue regeneration principles in endodontic surgery for induced chronic periodontic-endodontic lesions: a clinical, radiographic, and histologic evaluation. J Periodontol. 2005; 76:450–460.

18. Camelo MC, Nevins ML, Nevins M. Treatment of Class II furcations with autogenous bone grafts and e-PTFE membranes. Int J Periodontics Restorative Dent. 2000; 20:233–243.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download