1. Kasemo B, Lausmaa J. Aspects of surface physics on titanium implants. Swed Dent J Suppl. 1985. 28:19–36.
2. Zitter H, Plenk H Jr. The electrochemical behavior of metallic implant materials as an indicator of their biocompatibility. J Biomed Mater Res. 1987. 21:881–896.

3. Solar RJ, Pollack SR, Korostoff E. In vitro corrosion testing of titanium surgical implant alloys: an approach to understanding titanium release from implants. J Biomed Mater Res. 1979. 13:217–250.

4. Tengvall P, Lundstrom I. Physico-chemical considerations of titanium as a biomaterial. Clin Mater. 1992. 9:115–134.

5. Branemark PI, Adell R, Breine U, Hansson BO, Lindström J, Ohlsson A. Intra-osseous anchorage of dental prostheses. I. Experimental studies. Scand J Plast Reconstr Surg. 1969. 3:81–100.

6. Van Noort R. Titanium: the implant material of today. J Mater Sci. 1987. 22:3801–3811.

7. Albrektsson T, Hansson HA, Ivarsson B. Interface analysis of titanium and zirconium bone implants. Biomaterials. 1985. 6:97–101.

8. Feng B, Chen JY, Qi SK, He L, Zhao JZ, Zhang XD. Characterization of surface oxide films on titanium and bioactivity. J Mater Sci Mater Med. 2002. 13:457–464.
9. Neupane MP, Kim VK, Park IS, Lee MH, Bae TS. Characterization of surface oxide films and cell toxicity evaluations with a quenched titanium surface. Met Mater Int. 2008. 14:443–448.

10. Gilbert JL, Buckley CA, Lautenschlager EP. Titanium oxide film fracture and repassivation: the effect of potential, pH and aeration. 1996. Philadelphia: ASTM special technical publication.
11. Choi JW, Heo SJ, Koak JY, Kim SK, Lim YJ, Kim SH, et al. Biological responses of anodized titanium implants under different current voltages. J Oral Rehabil. 2006. 33:889–897.

12. Pilliar RM. Medical device materials. 2004. Materials Park: ASM International.
13. Sunny MC, Sharma CP. Titanium-protein interaction: changes with oxide layer thickness. J Biomater Appl. 1991. 6:89–98.

14. Byun C, Jang JW, Kim IT, Hong KS, Lee BW. Anatase-to-rutile transition of titania thin films prepared by MOCVD. Mater Res Bull. 1997. 32:431–440.

15. Barksdale J. Titanium, its occurrence, chemistry, and technology. Soil Sci. 1950. 70:414.

16. Lim YJ, Oshida Y, Andres CJ, Barco MT. Surface characterizations of variously treated titanium materials. Int J Oral Maxillofac Implants. 2001. 16:333–342.
17. Schrader ME. On adhesion of biological substances to low-energy solid-surfaces. J Colloid Interface Sci. 1982. 88:296–297.
18. Schakenraad JM, Busscher HJ, Wildevuur CR, Arends J. The influence of substratum surface free energy on growth and spreading of human fibroblasts in the presence and absence of serum proteins. J Biomed Mater Res. 1986. 20:773–784.

19. Anselme K, Bigerelle M. Statistical demonstration of the relative effect of surface chemistry and roughness on human osteoblast short-term adhesion. J Mater Sci Mater Med. 2006. 17:471–479.

20. Boyan BD, Lossdorfer S, Wang L, Zhao G, Lohmann CH, Cochran DL, et al. Osteoblasts generate an osteogenic microenvironment when grown on surfaces with rough microtopographies. Eur Cell Mater. 2003. 6:22–27.

21. Passeri G, Cacchioli A, Ravanetti F, Galli C, Elezi E, Macaluso GM. Adhesion pattern and growth of primary human osteoblastic cells on five commercially available titanium surfaces. Clin Oral Implants Res. 2010. 21:756–765.

22. van Oss CJ, Chaudhury MK, Good RJ. Monopolar surfaces. Adv Colloid Interface Sci. 1987. 28:35–64.

23. Anselme K. Osteoblast adhesion on biomaterials. Biomaterials. 2000. 21:667–681.

24. Vaquila I, Vergara LI, Passeggi MC, Vidal RA, Ferr J. Chemical reactions at surfaces: titanium oxidation. Surf Coat Technol. 1999. 122:67–71.

25. Jones P, Hockey JA. Infra-red studies of rutile surfaces. Part 2. Hydroxylation, hydration and structure of rutile surfaces. Trans Faraday Soc. 1971. 67:2679–2685.

26. Schwartz Z, Lohmann CH, Oefinger J, Bonewald LF, Dean DD, Boyan BD. Implant surface characteristics modulate differentiation behavior of cells in the osteoblastic lineage. Adv Dent Res. 1999. 13:38–48.

27. Gerstenfeld LC, Chipman SD, Glowacki J, Lian JB. Expression of differentiated function by mineralizing cultures of chicken osteoblasts. Dev Biol. 1987. 122:49–60.

28. Combe EC, Owen BA, Hodges JS. A protocol for determining the surface free energy of dental materials. Dent Mater. 2004. 20:262–268.

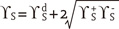
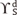 dispersive component (Lifshitz-van der Waals interactions)
dispersive component (Lifshitz-van der Waals interactions)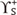 ,
, 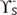 polar components (polar interactions, Lewis acid-base)
polar components (polar interactions, Lewis acid-base)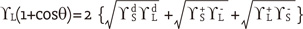




 PDF
PDF ePub
ePub Citation
Citation Print
Print












 XML Download
XML Download