Abstract
Purpose
This study was performed to establish an experimental rabbit model for single-stage maxillary sinus augmentation with simultaneous implant placement.
Methods
Twelve mature New Zealand white rabbits were used for the experiments. The rabbit maxillary sinuses were divided into 3 groups according to sinus augmentation materials: blood clot (BC), autogenous bone (AB), and bovine-derived hydroxyapatite (BHA). Small titanium implants were simultaneously placed in the animals during the sinus augmentation procedure. The rabbits were sacrificed 4 and 8 weeks after surgery and were observed histologically. Histomorphometric analyses using image analysis software were also performed to evaluate the parameters related to bone regeneration and implant-bone integration.
Results
The BC group showed an evident collapse of the sinus membrane and limited new bone formation around the original sinus floor at 4 and 8 weeks. In the AB group, the sinus membrane was well retained above the implant apex, and new bone formation was significant at both examination periods. The BHA group also showed retention of the elevated sinus membrane above the screw apex and evident new bone formation at both points in time. The total area of the mineral component (TMA) in the area of interest and the bone-to-implant contact did not show any significant differences among all the groups. In the AB group, the TMA had significantly decreased from 4 to 8 weeks.
Conclusions
Within the limits of this study, the rabbit sinus model showed satisfactory results in the comparison of different grafting conditions in single-stage sinus floor elevation with simultaneous implant placement. We found that the rabbit model was useful for maxillary sinus augmentation with simultaneous implant placement.
Implant placement in the posterior maxilla remains a challenge due to reduced bone volume, resulting from alveolar bone resorption and/or pneumatization of the sinus cavity. To overcome the problem of inadequate bone volume, sinus augmentation has been performed. Sinus augmentation can be achieved using one of the following procedures: the crestal approach using the osteotome technique [1-5] or one- or two-stage sinus floor elevation with a lateral approach [6-9]. Sinus floor elevation for dental implant placement has shown satisfactory results regardless of the surgical approach [10-16].
Various materials including autograft, allograft, xenograft, alloplast, and their combinations have been used for sinus augmentation. The fate of these grafts in the maxillary sinus has been investigated by many researchers. Because histologic investigation on human biopsies was restricted, animal models have been used. Watanabe et al. [17] used a rabbit maxillary sinus model for histologic investigation of the fate of autogenous particulated bone grafted in the maxillary sinus. Ever since the report by Watanabe et al. [17], the rabbit sinus model has also been used to evaluate various graft materials and grafting protocols including recombinant human bone morphogenetic protein-2 and autogenous bone (AB) [18], platelet-rich plasma (PRP) with an AB graft [19], mesenchymal stem cells/PRP and AB with PRP complexes [20], osteotome sinus elevation and simultaneous placement of porous surfaced dental implants [21], and tissue-engineered bone complex with alloplast and bone marrow stromal cells [22].
Those previous studies using a rabbit sinus model focused on the histologic changes of the grafted materials, and the surgical procedures performed were the first stage surgery of two-stage sinus floor elevation. However, one-stage sinus floor elevation with simultaneous implant placement is preferred to reduce the total treatment time in a clinical situation. At this time, no study using a rabbit model for one-stage sinus augmentation with simultaneous implant placement has been performed. The purpose of this study was to establish an experimental model for one-stage sinus floor elevation with simultaneous implant placement in rabbits. To evaluate the usefulness of the experimental model, the histologic changes of the augmented sinus cavity were observed and the histomorphometric measurements of several parameters related to the dental implant were also performed.
Twelve mature New Zealand white rabbits (aged 3 months, and weighing 3 to 3.5 kg) were used for the experiments. The animal research protocol was approved by the Institute of Laboratory Animal Resources, Seoul National University (SNU-090407-1). This study was designed as a prospective, randomized, controlled experiment. There were three experimental groups according to the sinus filling materials: the blood clot (BC) group (elevated sinus cavity filled with BC), the AB group (sinus augmented with AB), and the bovine-derived hydroxyapatite (BHA) group (sinus augmented with BHA [BioOss, Geistlich Biomaterials, Wolhusen, Switzerland]). Both sinuses of each rabbit were augmented with 2 different materials. A total of 24 maxillary sinuses from 12 animals were elevated, and a combination of 2 different filling materials was randomly assigned to each rabbit according to the blocked randomization method. Six rabbits were sacrificed after 4 weeks of healing, and the other six rabbits were sacrificed after 8 weeks.
The surgical procedure performed in this study was a modification of a surgical procedure previously performed by Watanabe et al. [17]. The position and size of the access window was the same, but the bony window was removed by a low speed carbide round bur instead of being infractured.
The animals were anesthetized with an intramuscular injection of xylazine (8.8 mg/kg body weight; Rompun, Bayer Korea, Seoul, Korea) and ketamine (35 mg/kg body weight; Yuhan, Seoul, Korea). The estimated surgical area was depilated on the cheek of each rabbit using an electric shaver. The top of the head area was also depilated when there was a need to harvest AB.
For the sinus augmentation, the surgical site was disinfected with betadine, and local anesthetics (2% lidocaine solution; Lignospan, Septodont, Lancaster, France) were administered. A skin incision was made in the cheek a few millimeters above the inferior border of the incisive bone and the maxilla. The subcutaneous tissue and the masseter muscle were divided to expose the maxillary periosteum, which was incised and elevated. A low speed carbide round bur was used to prepare the window (5×5 mm with the distal border located about 2 mm anterior to the mesial surface of the maxillary first molar) in the lateral antral wall of the maxilla with care not to perforate the antral membrane. The sinus membrane was elevated with a surgical curette, and then a 1.4 mm diameter, 6 mm long titanium mini-implant (14-AT-006, Jeil Medical Co., Seoul, Korea), which was made of grade V titanium alloy and had a smooth surface, was installed on the edentulous alveolar ridge (Fig. 1). The sinus space was augmented with one among the BC, the particulated AB, and the BHA according to the results of randomization. After augmentation, a single layer of collagen membrane (BioGide, Geistlich Biomaterials) was placed on the window, and the periosteum was closed tightly to secure the membrane with 4-0 chromic gut sutures (Woori Medical, Seoul, Korea). The skin was subsequently closed using 3-0 silk suture materials (Woori Medical).
The sinus augmentation procedures were as follows. For the BC group, about 1.5 mL of blood was collected during incision and flap elevation using two 1 mL insulin syringes. After completion of sinus membrane elevation, the collected blood was injected until the sinus cavity flooded. The volume of collected blood was sufficient to fill the elevated sinus space. The closure of the surgical site was delayed until the BC formation in the sinus cavity was identified. For the AB group, AB was harvested at the calvarium. The surgical site disinfection and local anesthesia were performed in the same manner mentioned above. After an incision was made following the application of a sagittal suture on the calvarium, the periosteal flaps were reflected. Two whole-depth calvarial bone blocks were carefully obtained from the parietal bone using an 8-mm internal diameter trephine bur (Ace Surgical Supply, Brockton, MA, USA) with copious saline irrigation. Extreme care was taken to avoid injury to the brain. The periosteum was sutured with 4-0 chromic gut (Woori Medical), and the skin was then sutured using 3-0 silk (Woori Medical). The bone blocks were particulated using a bone crusher (BCR-02, Osung MND Co., Seoul, Korea), and about 1 mL of bone particles was obtained. All of the AB particles were packed into the sinus cavity. For the BHA group, BHA granules 0.25 to 1 mm in size were used. The granules were tightly packed into the sinus cavity. About 0.5 g of BHA granules was inserted per sinus cavity.
After surgery, the animals were administered antibiotics (57 mg/kg; cefazolin sodium, Chong Kun Dang Pharmaceutical Co., Seoul, Korea) by an intramuscular injection twice a day for 2 days. Each rabbit was individually caged and received food and water.
Undecalcified histologic sections were prepared according to the sawing and grinding technique as previously described by Donath and Breuner [23]. The retrieved specimens were fixed in a 10% neutral-buffered formalin solution, dehydrated through a series of ethanol solutions of increasing concentrations, and embedded in embedding media (Technovit 7200, Exakt, Hamburg, Germany). The coronal sections encompassing the implant were sliced, ground with a cutting and grinding system (Exakt, Hamburg, Germany), and stained with basic fuchsin and methylene blue (Multiple Stain Solution, Polysciences, PA, USA).
Histologic examinations were conducted using a light microscope (Olympus BH-2, Olympus Optical, Osaka, Japan). The microscopic image from each slide was captured and saved to a computer for histomorphometric analysis. Measurements were carried out using an automated image analysis system (Tomoro Scope Eye 3.5 Image Analyzer, Techsan Digital Imaging, Seoul, Korea).
In each specimen, the area of interest (AOI) was virtually selected (Fig. 2A). The total area of mineralized component (TMA) and bone to implant contact (BIC) were measured in the AOI according to the following criteria.
AOI: the virtual rectangular box of which the vertical dimension was the distance from the peak of the 1st thread to the peak of the 4th thread and of which the horizontal dimension was 1 thread pitch distance from the valley of the thread (Fig. 2B).
TMA (%): the ratio of the area (Fig. 2C) of the newly formed bone and graft particles in the AOI to the total area of the AOI.
BIC (%): the ratio of the total perimeter of bone contact to the whole thread perimeter in the AOI.
In all of the animals, the sinus augmentation procedure with simultaneous implant placement was successfully performed without any significant problems. There was no sinus membrane perforation. All of the animals survived well until both examination points in time and the wounds also healed without any adverse reactions.
In the BC group (Fig. 3A), the sinus membrane descended below the level of the implant apex. Most of the space under the sinus membrane was composed of loose connective tissue and adipose tissue. The newly formed bone that originated from the original sinus floor (OSF) was observed around the lower half of the implant. However, bone-to-implant contact was hardly seen. In the AB group (Fig. 3B), the sinus membrane was observed around the implant apex. The augmented sinus cavity was filled with grafted bone particles and newly formed woven bone. New bone formation was mainly found near the screw surface, and bone-to-implant contact was observed. In the BHA group (Fig. 3C), the sinus membrane was well supported above the level of the implant apex. Newly formed bone had been deposited on the surface of the BHA particles and the implant surface. Bone-to-implant contact was also found.
In the BC group (Fig. 4A), the drooping sinus membrane descended to the level of the apical third of the implant. New bone formation was mainly observed at the lower half of the screw, which extended from the OSF. The space between the newly formed bone and sinus membrane was filled mainly with loose connective tissue and adipose tissue. The lateral window area was still open and was penetrated by loose connective tissue and adipose tissues. Bone-to-implant contact was found only in the lower half of the implant. In the AB group (Fig. 4B), the sinus membrane was well supported near the level of the implant apex. A large amount of new bone was deposited on the implant surface. Adipose tissues were also found in the central region of the augmented space. The lateral window was coalesced by new bone formation. Bone-to-implant contact was evident along the whole implant. The BHA group (Fig. 4C) showed that the sinus membrane was maintained above the level of the implant apex. Newly formed bone was found on the BHA surface and the space between the particles. The lateral window area was filled with BHA particles and newly formed bone. Evident bone-to-implant contact was also observed.
Table 1 presents the histomorphometric results from the experiment. The TMA of all the groups ranged from 23.2 to 38.1% at 4 weeks and from 25.2 to 46.0% at 8 weeks. At 8 weeks, the AB group showed a decrease in the TMA, whereas the other two groups showed an increase, when compared to the 4 week results. The decrease in the TMA of the AB group between 4 weeks and 8 weeks was statistically significant (P=0.028).
The average BIC of all the groups ranged from 11.3 to 29.0% at 4 weeks and from 22.6 to 33.5% at 8 weeks. The BIC was also the greatest in the BHA group at both points in time. There were no significant differences between the groups at either point in time or between the 2 points in time within any group.
Animal models have been widely used in dental research to understand the nature of oral disease, to investigate the effects of different treatment modalities, and to test materials such as dental implants and bone grafts. According to a recent review [24], rabbits have been used for establishing a new animal experimental model. The rabbit experimental model of maxillary sinus augmentation was firstly introduced by Watanabe et al. [17]. They stated that the advantages of the rabbit model were low cost, ease of experimentation, and easy distinction of membrane perforation. After their introduction of this model, successful experiments using the rabbit sinus model were performed by others. However, the previous studies had mainly focused on the response of various graft materials in the sinus without implant placement [18-20,22]. As a result, they assessed new bone formation in a qualitative manner. Meanwhile, in the present study, the rabbit experimental model for maxillary sinus augmentation with simultaneous implant placement was successfully performed in order to compare different grafting materials. By employing a mini-implant as a reference for the measurements, space maintenance, new bone formation, and bone-to-implant contact could be successfully analyzed in a quantitative manner.
Some technical limitations existed during histologic processing despite the positive results of this study. During sectioning, minor errors may have occurred because it was difficult to achieve sawing and grinding exactly through the center of the implant. Furthermore, due to the narrow diameter of the mini-implant, it was difficult to obtain more than one section or plane per specimen. To minimize sectioning errors, the TMA and BIC were analyzed in selected AOIs. Considering that the BC group showed limited new bone formation near the OSF and given the distance from the mini-implant to the medial wall of the sinus, the vertical dimension of the AOI was set as the distance from the peak of the 1st thread to the peak of the 4th thread and the horizontal dimension of the AOI was set as 1 thread pitch distance between the first and second thread in each section. Finally, the new bone area and BIC were also represented as a percentage degree instead of actual measurement dimensions. By employing the AOI, the differences in the amount of new bone formation and the bone-to-implant contact ratio after the use of various graft materials could be successfully compared.
The histologic changes after AB grafting in a rabbit sinus were observed by Watanabe et al. [17]. In their experiment, the rabbit sinus floor was elevated, and the space was filled with AB from the iliac crest. They observed active new bone formation until 4 weeks. However, they could see matured bone with a large central portion of marrow space filled with fat cells and surrounding continuous cortical bone. In the present study, the AB group showed new bone formation as well as the resorption of the grafted bone particles at 4 weeks. At 8 weeks, the AB group showed well organized bone healing. These findings are similar to the observations of Watanabe and colleagues, although there was a difference in the donor site.
For sinus augmentation, the slow resorption property of BHA may play a role in space maintenance. In addition, rapidly progressing degradation would endanger the stability of the implant site. In the present study, slow resorption of BHA and its effectiveness in maintaining the augmented space were also observed. The BHA group allowed in-growth of newly formed bone between the BHA particles. Meanwhile, resorption and/or replacement of BHA was hardly observed until 8 weeks. In the literature, the resorption of BHA has been a controversial subject. Schlegel and Donath [25] reported the retention of BHA particles after 6 years, and even longer in humans. They reported the in-growth of newly formed bone in the BHA scaffold and no clinical signs of BHA resorption. Meanwhile, McAllister et al. [26] reported that BHA grafted in the chimpanzee sinus had been resorbed and replaced by vital bone.
Handschel et al. [27] performed a meta-analysis of sinus elevation with various grafting materials in humans. In their report, the total bone volume (TBV) of AB without any additional grafting material had decreased as time passed, while that of BHA, BHA with AB, and β-tricalclium phosphate showed a steep increase. In addition, TBV of AB only was significantly higher than that of others until 9 months, but the difference among the graft materials was lost after 9 months. In the present study, the AB group showed a decrease in TMA from 4 to 8 weeks, while that of the BHA group increased over time. Our group has reported on bone reaction to BHA alone for maxillary sinus floor augmentation in humans [28]. In that study, we observed an increase in the percentage of bone in the grafted area from 6 to 12 months without a concomitant decrease in the proportion of BHA. However, the question still remains as to what role AB plays in the healing process and whether it can be completely replaced with a bone substitute. Hallman et al. [29] evaluated implant integration in the posterior maxilla after sinus floor augmentation with AB, BHA, or a 20:80 mixture of the two. Their histomorphometric analysis showed no differences in the new bone area among the 3 groups, indicating that an AB graft can be substituted with BHA to 80% or 100% when used in sinus floor augmentation. Their results suggest that the effect of adding AB remains unclear.
The BIC has been regarded as a parameter for the biocompatibility of implant material. Johansson and Albrektsson [30] selected the 3 best consecutive threads of an implant for measurements of the BIC. In the present study, we also employed a similar method to analyze the implant response to 3 different graft conditions. At 4 and 8 weeks, the BHA group showed a slightly better BIC value than the AB group. This finding means that implant placement in 100% BHA for a sinus augmentation procedure resulted in a just as predictable integration as that of AB. Hallman et al. [29] also reported similar results in their human subjects. In their histomorphometric analysis, the BIC did not differ between the AB group and the BHA group.
In conclusion, the present study demonstrated that a differential response of the bone graft materials and therapies related to dental implants in the maxillary sinus could be established using the rabbit experimental model. In addition, it demonstrated that AB and BHA are both effective graft materials for sinus augmentation.
Figures and Tables
 | Figure 1An experimental site. The lateral window position and installed mini-implant (arrow) are shown in a schematic drawing (A). The sinus membrane, original sinus floor, and mini-implant (arrow) are seen in the photograph (B). |
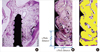 | Figure 2Area of interest (AOI). The AOI (A) was virtually selected in each specimen. B and C are the magnified view of AOI from A. The vertical dimension of AOI was set as the distance from the peak of 1st thread to the peak of 4th thread and the horizontal dimension was 1 thread pitch distance from the valley of the thread (B). In C, the area of newly formed bone and graft particles was yellow-highlighted for histomorphometric analysis. Basic fuchsin & methylene blue stain (A, ×20; B and C, ×40). |
 | Figure 3Photographs of 4 weeks (basic fuchsin & methylene blue stain, ×20). Yellow line indicates original sinus floor (OSF). (A) The BC group, the sinus membrane descended below the level of implant apex. New bone formation was restricted near OSF. (B) The AB group, the sinus membrane observed beside the implant apex. The grafted autogenous bone particles were found. (C) The BHA group, the sinus membrane was supported by BHA particles above the implant apex. New bone was deposited on BHA particles. The sinus membrane was indicated by arrowheads. BC group: blood clot filled group, AB group: autogenous bone filled group, BHA group: bovine-derived hydroxyapatitie filled group, NB: newly formed bone. |
 | Figure 4Photographs of 8 weeks (basic fuchsin & methylene blue stain, ×20). Yellow line indicates original sinus floor (OSF). (A) The BC group, the sinus membrane descended below the implant apex. (B) The AB group, the sinus membrane retained over the implant apex. Note the newly formed bone and central marrow spaces. (C) The BHA group, the sinus membrane was supported by BHA particles and retained above the screw apex. New bone was found between BHA particles. The sinus membrane was indicated by arrowheads. BC group: blood clot filled group, AB group: autogenous bone filled group, BHA group: bovine-derived hydroxyapatite filled group, NB: newly formed bone, BHA: bovine-derived hydroxyapatite. |
ACKNOWLEDGEMENTS
This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MEST) (No. 2011-0027790).
References
2. Summers RB. A new concept in maxillary implant surgery: the osteotome technique. Compendium. 1994. 15:152154–156. 158

3. Rosen PS, Summers R, Mellado JR, Salkin LM, Shanaman RH, Marks MH, et al. The bone-added osteotome sinus floor elevation technique: multicenter retrospective report of consecutively treated patients. Int J Oral Maxillofac Implants. 1999. 14:853–858.

4. Fugazzotto PA. The modified trephine/osteotome sinus augmentation technique: technical considerations and discussion of indications. Implant Dent. 2001. 10:259–264.

5. Chen L, Cha J. An 8-year retrospective study: 1,100 patients receiving 1,557 implants using the minimally invasive hydraulic sinus condensing technique. J Periodontol. 2005. 76:482–491.
6. Boyne PJ, James RA. Grafting of the maxillary sinus floor with autogenous marrow and bone. J Oral Surg. 1980. 38:613–616.
7. Lazzara RJ. The sinus elevation procedure in endosseous implant therapy. Curr Opin Periodontol. 1996. 3:178–183.

8. Peleg M, Mazor Z, Chaushu G, Garg AK. Sinus floor augmentation with simultaneous implant placement in the severely atrophic maxilla. J Periodontol. 1998. 69:1397–1403.

9. Zitzmann NU, Scharer P. Sinus elevation procedures in the resorbed posterior maxilla. Comparison of the crestal and lateral approaches. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998. 85:8–17.
10. Jensen OT, Shulman LB, Block MS, Iacono VJ. Report of the Sinus Consensus Conference of 1996. Int J Oral Maxillofac Implants. 1998. 13:Suppl. 11–45.
11. Peleg M, Mazor Z, Garg AK. Augmentation grafting of the maxillary sinus and simultaneous implant placement in patients with 3 to 5 mm of residual alveolar bone height. Int J Oral Maxillofac Implants. 1999. 14:549–556.

12. Mayfield LJ, Skoglund A, Hising P, Lang NP, Attstrom R. Evaluation following functional loading of titanium fixtures placed in ridges augmented by deproteinized bone mineral. A human case study. Clin Oral Implants Res. 2001. 12:508–514.

13. Fugazzotto PA. Immediate implant placement following a modified trephine/osteotome approach: success rates of 116 implants to 4 years in function. Int J Oral Maxillofac Implants. 2002. 17:113–120.

14. Wallace SS, Froum SJ. Effect of maxillary sinus augmentation on the survival of endosseous dental implants. A systematic review. Ann Periodontol. 2003. 8:328–343.

15. Emmerich D, Att W, Stappert C. Sinus floor elevation using osteotomes: a systematic review and meta-analysis. J Periodontol. 2005. 76:1237–1251.

16. Ferrigno N, Laureti M, Fanali S. Dental implants placement in conjunction with osteotome sinus floor elevation: a 12-year life-table analysis from a prospective study on 588 ITI implants. Clin Oral Implants Res. 2006. 17:194–205.

17. Watanabe K, Niimi A, Ueda M. Autogenous bone grafts in the rabbit maxillary sinus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999. 88:26–32.
18. Wada K, Niimi A, Watanabe K, Sawai T, Ueda M. Maxillary sinus floor augmentation in rabbits: a comparative histologic-histomorphometric study between rhBMP-2 and autogenous bone. Int J Periodontics Restorative Dent. 2001. 21:252–263.
19. Butterfield KJ, Bennett J, Gronowicz G, Adams D. Effect of platelet-rich plasma with autogenous bone graft for maxillary sinus augmentation in a rabbit model. J Oral Maxillofac Surg. 2005. 63:370–376.
20. Ohya M, Yamada Y, Ozawa R, Ito K, Takahashi M, Ueda M. Sinus floor elevation applied tissue-engineered bone. Comparative study between mesenchymal stem cells/platelet-rich plasma (PRP) and autogenous bone with PRP complexes in rabbits. Clin Oral Implants Res. 2005. 16:622–629.
21. Rahmani M, Shimada E, Rokni S, Deporter DA, Adegbembo AO, Valiquette N, et al. Osteotome sinus elevation and simultaneous placement of porous-surfaced dental implants: a morphometric study in rabbits. Clin Oral Implants Res. 2005. 16:692–699.
22. Sun XJ, Zhang ZY, Wang SY, Gittens SA, Jiang XQ, Chou LL. Maxillary sinus floor elevation using a tissue-engineered bone complex with OsteoBone and bMSCs in rabbits. Clin Oral Implants Res. 2008. 19:804–813.
23. Donath K, Breuner G. A method for the study of undecalcified bones and teeth with attached soft tissues. The Sage-Schliff (sawing and grinding) technique. J Oral Pathol. 1982. 11:318–326.

24. Dannan A, Alkattan F. Animal models in periodontal research: a mini-review of the literature. Internet J Vet Med [Internet]. 2008. cited 2009 Aug 12. 5:about 3. Available from: http://www.ispub.com/journal/the-internet-journal-of-veterinary-medicine/volume-5-number-1/animal-models-in-periodontal-research-a-mini-review-of-the-literature.html#sthash.Jpt0qK5R.dpbs.

25. Schlegel AK, Donath K. BIO-OSS: a resorbable bone substitute? J Long Term Eff Med Implants. 1998. 8:201–209.
26. McAllister BS, Margolin MD, Cogan AG, Buck D, Hollinger JO, Lynch SE. Eighteen-month radiographic and histologic evaluation of sinus grafting with anorganic bovine bone in the chimpanzee. Int J Oral Maxillofac Implants. 1999. 14:361–368.
27. Handschel J, Simonowska M, Naujoks C, Depprich RA, Ommerborn MA, Meyer U, et al. A histomorphometric meta-analysis of sinus elevation with various grafting materials. Head Face Med. 2009. 5:12.

28. Lee YM, Shin SY, Kim JY, Kye SB, Ku Y, Rhyu IC. Bone reaction to bovine hydroxyapatite for maxillary sinus floor augmentation: histologic results in humans. Int J Periodontics Restorative Dent. 2006. 26:471–481.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download