Abstract
Purpose
The osseointegration around titanium mini-implants installed in macroporous biphasic calcium phosphate (MBCP) blocks was evaluated after incubation with recombinant human bone morphogenetic protein-2 (rhBMP-2) in an ectopic subcutaneous rat model.
Methods
Mini-implants (φ1.8×12 mm) were installed in MBCP blocks (bMBCPs, 4×5×15 mm) loaded with rhBMP-2 at 0.1 mg/mL, and then implanted for 8 weeks into subcutaneous pockets of male Sprague-Dawley rats (n=10). A histomorphometric analysis was performed, and the bone-to-implant contact (BIC) and bone density were evaluated.
Recombinant human bone morphogenetic protein-2 (rhBMP-2) is a member of the bone morphogenetic protein (BMP) family that is involved in de novo bone induction. In both preclinical and clinical settings, rhBMP-2 has been demonstrated to induce bone formation in a variety of conditions including supra-alveolar periodontal defects [1], three-wall intrabony periodontal defects [2], cleft palate defects [3], class III alveolar defects [4], calvarial defects [5,6], and ectopic subcutaneous pockets [7]. The advent of dental implants, which are gradually replacing conventional prosthetic treatments, has recently expanded the application of rhBMP-2 to alveolar ridge augmentation [8-10] and sinus floor elevation [11], for which inadequate bone volume might adversely affect the long-term prognosis of dental implants [12].
Currently, various animal models exist for both implant osseointegration and rhBMP-2-enhanced bone regeneration including the ectopic rat model [13,14], supra-alveolar peri-implant model [15-20], and mandible/tibia model [21,22]. Among these, the ectopic rat model is one of the most extreme in vivo osteoinduction models that completely exclude the osteoconductive factors from the surrounding native bone, thereby enabling the evaluation of the effect of rhBMP-2 alone. This model is also considered to be one of the best models for evaluating the bone-forming potential of rhBMP-2 in combination with various implant surface treatments. Previous studies have already demonstrated that rhBMP-2 can induce significant new bone regeneration around titanium implants transplanted into the subcutaneous pouches of immunocompromised mice [13,14]. However, such studies have ignored the crucial role of the scaffold, which allows cell invasion for osteoinduction and retains rhBMP-2 at the implantation site; tissue regeneration should always be considered in terms of basic factors such as cells, signals, and scaffolds [23]. Furthermore, the biomaterials used as a scaffold could duplicate the clinical characteristics of implants installed in alveolar bony ridges as well as in microenvironments involving osteoconduction or osteoinduction.
Various materials have been utilized in combination as carrier materials for rhBMP-2 including hydroxyapatite, absorbable collagen sponge, β-tricalcium phosphate, and macroporous biphasic calcium phosphate (MBCP). We previously evaluated the potential of MBCP block (bMBCP) for the application of rhBMP-2 [24] and demonstrated significant new bone formation. In the present study, we evaluated bone healing and osseointegration around titanium mini-implants installed in bMBCPs treated with Escherichia coli-expressed rhBMP-2 to induce osseointegration in an ectopic subcutaneous rat model from a genetically homogeneous strain. This approach constituted a proof-of-concept study of osseointegration and ectopic bone formation in the rhBMP-2/bMBCP system that used a standardized experimental model to evaluate the sole effect of rhBMP-2 on both osseointegration and bone formation. To our knowledge, this is the first study to demonstrate ectopic osseointegration and bone formation using the rhBMP-2/bMBCP system.
As previously described, rhBMP-2 was expressed in E. coli at the Research Institute of Cowellmedi (Busan, Korea) [25]. Briefly, total RNA from human osteosarcoma cells was reverse-transcribed with reverse transcriptase (Gibco BRL, Grand Island, NY, USA). The cDNA encoding of the mature form of the BMP-2 protein was amplified via polymerase chain reaction. The cDNA of human BMP-2 (hBMP-2) was then subcloned into a pRSET(A) vector (Invitrogen, Paisley, UK) to produce the pRSET(A)/hBMP-2 expression vector, which was used to transform the E. coli BL21(DE3) strain. A high-cell density cultivation of E. coli was crushed twice in a French press and then centrifuged. The pellet was then resuspended at 25 mg wet weight/mL in a suspension buffer (20 mM Tris-HCl [pH 8.5], 0.5 mM ethylenediaminetetraacetic acid [EDTA], and 2% v/v Triton X-100), and then centrifuged again. The inclusion bodies (pellets) were resuspended and incubated overnight.
For the in vitro dimerization, the solubilized rhBMP-2 was incubated in a renaturation buffer (0.5 M guanidine-HCl, 50 mM Tris-HCl [pH 8.5], 0.75 M N-cyclohexyl-2-aminoethanesulfonic acid, 1 M NaCl, 5 mM EDTA, and 3 mM total glutathione). Purification of the active rhBMP-2 (dimer) was performed with a heparin column (Heparin Sepharose 6 Fast Flow, GE Healthcare, Milwaukee, WI, USA). The active rhBMP-2 protein was eluted and separated using a stepped NaCl gradient (0.15, 0.3, and 0.5 M). Finally, this rhBMP-2 was reconstituted and diluted in a buffer to a concentration of 0.1 mg/mL.
An experiment to determine the release kinetics of rhBMP-2 in vitro was conducted using a slight modification of a previously described method [26]. Briefly, bMBCPs (n=5) loaded with 0.1 mL of rhBMP-2 solution were placed into 2 mL microcentrifuge tubes containing 1.0 mL of phosphate-buffered saline solution (pH 7.4) and 0.02% (w/v) sodium azide. The tubes were incubated at 37℃ with continuous agitation. The supernatant medium was collected and completely replaced with a fresh buffer solution at the scheduled time points. The amount of rhBMP-2 in the supernatant was determined by a competitive indirect enzyme-linked immunosorbent assay (ELISA) for rhBMP-2 (Human BMP-2 R&D Systems, Minneapolis, MN, USA) according to the manufacturer's protocol. The absorbance of the samples was read at 450 nm using an ELISA plate reader (Model 680, Bio-Rad Laboratories Inc., Hercules, CA, USA). The amount of rhBMP-2 was calculated with the aid of a calibration curve (Fig. 1).
Titanium-surfaced mini-implants (1.8 mm in diameter and 12 mm long; Cheil Pharma and Instrument, Seoul, Korea) were placed into the bMBCPs (4×5×15 mm; Biomatlante, Vigneux de Bretagne, France). The blocks had one longitudinal hole to allow for implant placement and three horizontal holes to facilitate bone healing (Fig. 2). The diameter of each horizontal hole was 2 mm, while that of the vertical hole was 1.5 mm. Implants were installed manually along the vertical hole, with subsequent confirmation that they did not rotate after fixation. The bMBCP was treated overnight with either a phosphate buffer solution (control group) or rhBMP-2 solution at 0.1 mg/mL (test group) before transplantation.
Ten male Sprague-Dawley rats (body weight 250 to 300 g) were assigned to either the test or control group (n=5 animals per group). The animals were maintained in plastic cages in a room with a 12-hour day/night cycle, an ambient temperature of 21℃, and ad libitum access to water as well as a standard laboratory pellet diet. Animal selection, management, surgical protocol, and preparation followed routines approved by the Institutional Animal Care and Use Committee of Yonsei Medical Center in Seoul, Korea.
The animals were placed under general anesthesia using an intramuscular injection (5 mg/kg body weight) of ketamine hydrochloride (Ketalar, Yuhan, Seoul, Korea). The surgical site was shaved and scrubbed with iodine, and a vertical incision was then made in the skin of the back. After flap reflection, a subcutaneous pocket was prepared by blunt dissection, and a bMBCP with a titanium implant, treated or untreated with rhBMP-2, was implanted into the pocket. The skin was sutured for primary closure with 4-0 Vicryl sutures (Polyglactin 910 braided absorbable suture, Ethicon, Johnson & Johnson, Edinburgh, UK). The animals were sacrificed after a healing period of 8 weeks, and samples were later obtained for histological analysis.
After the bMBCP transplants were retrieved from the animals after necropsy, clinical photographs were taken (Fig. 3). Block samples were fixed in a 10% formalin solution, dehydrated in an ascending graded ethanol series (70%, 80%, 90%, 95%, and 100%) and pure acetone, and then impregnated and embedded in glycol methylmethacrylate. Polymerization was achieved by increasing the temperature from 20℃ to 80℃. Each resin block was cut into two sections (15 µm thickness) along the longitudinal axis of the biopsy sample using an internal circular diamond saw (Leitz, Wetzlar, Germany) [25]. After staining with hematoxylin and eosin (H&E), all of the samples were examined with the aid of light and polarized-light microscopy (BX50, Olympus Co., Tokyo, Japan), as well as scanning electron microscopy (SEM; S-4300, Hitachi, Tokyo, Japan). The SEM observations were performed after the sections were polished and sputtered with gold palladium, using secondary and back-scattered electrons at 15 kV (Leo 1450VP, Zeiss, Germany). The most central section from each block was selected to compare the findings between the groups. New bone formation and osseointegration in H&E-stained histological slides were analyzed quantitatively using an image analysis system (Image-Pro Plus; Media Cybernetics, Silver Spring, MD, USA). The following parameters were measured: 1) Bone-to-implant contact (BIC): The length of osseointegration that was along the thread profile and in direct contact with the newly formed bone was calculated for both sides of the implant on each of the two central sections obtained from each implant; 2) Bone density within the implant threads: The area of the newly formed bone within the implant threads was measured. The value of each was expressed as a percentage.
The rhBMP-2 release kinetics of bMBCP were evaluated in vitro (Fig. 1). Approximately 80% of the loaded rhBMP-2 was released during the first 4 days. However, rhBMP-2 was continuously released for up to 2 weeks. Clinical healing was uneventful. In the test group, the horizontal holes became completely filled with hard collagenous tissues that were firmly attached to the block (Fig. 3A). Small diameter blood vessels were integrated within the dense fibrous tissue. The collagenous tissue could not be easily rubbed off from the bMBCP surface, suggesting highly increased tissue integration. Conversely, loosely formed connective tissue was formed along the bMBCP block of the control group, which was easily washed off (Fig. 3B).
The general pattern of bone formation was favorable in the test group, which was in line with the results from previous studies [24,27,28]. Newly formed bone stained with H&E exhibited a high-density, predominantly lamellated bone pattern along the implant surface and within the micropores of the bMBCP. This pattern was different from the control group, in which no mineralized tissue had formed. The distribution of the newly formed bone was equally observed along the implant and bMBCP surfaces, while the bridging pattern between the implant and bMBCP surfaces was clearly evident. The newly formed bone did not appear to be homogeneous and exhibited cement lines separating areas of bone that had been deposited at different times. The new bone appeared to be well mineralized and had numerous lacunae. Numerous osteocytes were observed, as well as a few osteoblasts that were aligned along the surface of the newly formed bone.
The results of the histomorphometric analysis are shown in Table 1. The percentage of direct BIC was 41.23±4.13% (mean±standard deviation) in the test group (n=5), while the control group exhibited no bone formation along the titanium mini-implants (n=5). The bone density in the test group was 33.47±5.73%.
Polarized-light microscopy was used to evaluate the patterns of lamellation in the newly formed bone, which is considered to be an important hallmark of bone maturation. In the treated group, groups of mineralized collagen fibers with numerous osteocytes were clearly observed under polarized light (Fig. 4C), while there appeared to be new bone formation in direct contact with the bMBCP and implant surfaces. Conversely, no mineralized tissue was found in the control group (Fig. 4F).
The back-scattered SEM images confirmed that the area of newly formed bone was significantly larger in the rhBMP-2-treated group than in the untreated control group 8 weeks after transplantation (Fig. 5). Newly formed bone was generally observed within the bMBCP pores and along the implant surface, appearing as light-gray areas. However, no mineralized tissue was observed in the sections from the control group. Collectively, mineralized bony tissue on the implant and bMBCP surfaces (including osteocytes, a Haversian system, and cement lines) was observed exclusively in the test group both during polarized light microscopy and in back-scattered SEM images.
To the best of our knowledge, this is the first report of an ectopic osseointegration analysis model based on titanium mini-implants and the rhBMP-2/bMBCP system, which closely simulated clinical parameters. We also evaluated the true osseointegration potential of rhBMP-2 by excluding other contributing factors. This study confirmed the feasibility of this evaluation model and the new bone formation potential of rhBMP-2. The results suggest that candidates for bone induction and techniques for new bone formation and osseointegration can be analyzed without experimental errors using this model.
The present study achieved clinically relevant amounts of BIC and an adequate density of newly formed bone within a relatively short period of time. This finding is in agreement with previous results, suggesting that the mechanism of new bone formation involves initiation of the osteoinduction cascade by MBCP at the local site and support for osteoinductive capacity by rhBMP-2 [24,27,29]. The potential of BMPs to induce new bone formation is crucially dependent on the characteristics of the carrier [30,31]. Moreover, it has been reported that the surface microstructure plays an important role in the osteoinductive effect of biomaterials [32]; the microstructure may allow biological apatite to precipitate and induce cells to attach to the surfaces of biomaterials. In particular, micropores with diameters smaller than 5 µm might serve as nucleation sites for biological apatite precipitation because they can facilitate ionic exchange with body fluids [33]. Additionally, micropores at the MBCP surface may favor the adsorption and entrapment of rhBMP-2, which has a high affinity for calcium phosphates [34]. As such, this microenvironment can enhance the adhesion and differentiation of progenitor cells into osteoblasts. Our results demonstrate that bMBCP can potentially mimic the clinical healing environment of an implant installed in the alveolar ridge.
In this study, newly formed bone was generally observed on implant surfaces and within the vertical and horizontal holes. A possible mechanism underlying this phenomenon appears to be related to the specific microenvironment of bMBCP. It has been reported that microparticles or ionic molecules are released from MBCP during the initial healing period [35] and that the released microparticles may induce the local release of inflammatory cytokines. This release of cytokines stimulates circulating stem cells to repopulate on the MBCP surface and differentiate into osteoblasts, thereby producing bone tissue. In our study, the sustained release of rhBMP-2 in the bMBCP for a relevantly long period may have induced these osteoblastic differentiation cascades. Even though bone formation and osseointegration were evenly distributed, more bone formation was observed in the vertical hole of the implant apex. We postulated that the osteoinductive properties were favorably enhanced by the specific microenvironment in this area, although additional studies are required to fully address this phenomenon.
Previous studies have demonstrated that carrier type can influence the effectiveness of rhBMP-2-induced bone formation, with several types of carrier systems having been suggested for the clinical use of rhBMPs [7,27,32,33,36,37]. MBCP is reportedly one of the most effective carrier systems for rhBMPs [29] and is composed of hydroxyapatite and tricalcium phosphate at a ratio of 60:40. MBCP also exhibits an excellent capacity for bone conduction and induction [34,35], which is accelerated when incorporated with rhBMP-2 [38,39]. Although we did not observe any ectopic bone formation in the control group in this study, other reports have described MBCP-induced ectopic new bone formation [34,35]. Particularly, excellent bone induction was observed when a powdered type of MBCP was used as an rhBMP-2 carrier, which shows its potential for future clinical application.
Research into the effects of rhBMPs on implant osseointegration has focused mainly on the alveolar bone defect model in canines. However, an orthotopic model in canines is difficult to standardize because of the individuality of the animals as well as the different healing patterns and variations in both bone quantity and quality, which may be associated with different healing vectors. Furthermore, most defect morphologies cannot exclude the contribution of each patient's innate healing potential. Therefore, it has been difficult to analyze the isolated induction capacity of rhBMP-2 on osseointegration and bone regeneration. The results of the present study, which involved a genetically identical rat strain, revealed that favorable ectopic new bone formation and osseointegration were possible using an rhBMP-2/bMBCP system. In addition, minimal bone formation was observed in the control group, indicating that this evaluation model was very standardized and reliable as a result of the exclusion of innate bone forming potential; this suggests that our standardized model was a good candidate for the analysis of orthotopic implant osseointegration.
Within the limitations of this study, the histology results, the polarized-light microscopy, and SEM analyses all demonstrated that impregnation of rhBMP-2 into a bMBCP significantly induced osteoinductive activity and enhanced osseointegration in subcutaneous rat tissues at 8 weeks. The titanium mini-implant and rhBMP-2/bMBCP system can be used to evaluate isolated healing characteristics of bone regeneration and osseointegration resulting from rhBMP-2, while excluding the innate healing factors from the host. We postulate that this specific model could be widely used in the process of developing new implant surfaces.
In conclusion, this model represents a more standardized tool for analyzing osseointegration and bone healing along the implant surface and bMBCP that excludes various healing factors derived from selected animals and defect models.
Figures and Tables
Figure 1
Kinetics of recombinant human bone morphogenetic protein-2 (rhBMP-2) release from macroporous biphasic calcium phosphate block observed in vitro. Sustained release of rhBMP-2 was observed for up to 2 weeks.

Figure 2
Clinical photograph of a macroporous biphasic calcium phosphate block (bMBCP) and titanium mini-implant installation. One vertical hole and three horizontal holes are present in the bMBCP. The titanium mini-implant was installed manually.
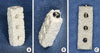
Figure 3
Clinical photograph of a macroporous biphasic calcium phosphate block (bMBCP) retrieved after necropsy. (A) High-density connective tissue formation was observed with infiltration of microvessels in the test group (recombinant human bone morphogenetic protein-2/bMBCP). (B) Loose connective tissue, which was easily wiped off, dominantly formed over the bMBCP in the control group (phosphate buffer solution/bMBCP).

Figure 4
Histological overview of the retrieved titanium mini-implants and recombinant human bone morphogenetic protein-2/macroporous biphasic calcium phosphate blocks (rhBMP-2/bMBCPs) or phosphate buffer solution/bMBCPs (H&E staining). (A, B) The rhBMP-2-treated test group presenting significant new bone formation along the implant surface and within the bMBCP (A, ×40; B, ×200). (C) The newly formed bone exhibited a lamellated pattern under polarized-light microscopy, suggesting the occurrence of waves of differentiated bone formation over time (×200). (D, E) The untreated control group demonstrated no mineralized tissue formation along the implant surface or within the bMBCP (D, ×40; E, ×200). (F) No mineralized tissue was observed under polarized-light microscopy in the control tissue (×200). Ti: titanium mini-implant, M: bMBCP, NB: new bone.
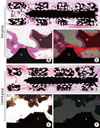
Figure 5
(A) Scanning electron microscopy (SEM) images of the test group (recombinant human bone morphogenetic protein-2/macroporous biphasic calcium phosphate blocks [bMBCPs]) 8 weeks after subcutaneous implantation in rats (×50). A higher magnification of the boxed area is shown in B. New bone formation is evident along the implant surface. The bMBCP is revealed as dark-gray areas, while MBCP and the titanium mini-implant appear as light-gray and white, respectively. (B) A higher magnification view of the boxed area clearly shows newly formed bone tissue (×100). The yellow arrowheads indicate the obvious formation of NB. (C) The SEM images of the control group (phosphate buffer solution/bMBCP) (×50). (D) Polarized-light microscopy showing the absence of mineralized tissue (×100). Ti: titanium mini-implant, M: bMBCP, NB: new bone.

ACKNOWLEDGEMENTS
This study was supported by a grant of the Korea Health technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A100443). We thank Ms. J. M. Lee for providing us with excellent graphics.
References
1. Wikesjo UM, Guglielmoni P, Promsudthi A, Cho KS, Trombelli L, Selvig KA, et al. Periodontal repair in dogs: effect of rhBMP-2 concentration on regeneration of alveolar bone and periodontal attachment. J Clin Periodontol. 1999. 26:392–400.

2. Blumenthal NM, Koh-Kunst G, Alves ME, Miranda D, Sorensen RG, Wozney JM, et al. Effect of surgical implantation of recombinant human bone morphogenetic protein-2 in a bioabsorbable collagen sponge or calcium phosphate putty carrier in intrabony periodontal defects in the baboon. J Periodontol. 2002. 73:1494–1506.

3. Boyne PJ, Nath R, Nakamura A. Human recombinant BMP-2 in osseous reconstruction of simulated cleft palate defects. Br J Oral Maxillofac Surg. 1998. 36:84–90.

4. Barboza EP, Duarte ME, Geolas L, Sorensen RG, Riedel GE, Wikesjo UM. Ridge augmentation following implantation of recombinant human bone morphogenetic protein-2 in the dog. J Periodontol. 2000. 71:488–496.

5. Jung JH, Yun JH, Um YJ, Jung UW, Kim CS, Choi SH, et al. Bone formation of Escherichia coli expressed rhBMP-2 on absorbable collagen block in rat calvarial defects. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011. 111:298–305.

6. Jang JW, Yun JH, Lee KI, Jang JW, Jung UW, Kim CS, et al. Osteoinductive activity of biphasic calcium phosphate with different rhBMP-2 doses in rats. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012. 113:480–487.

7. Kim CS, Kim JI, Kim J, Choi SH, Chai JK, Kim CK, et al. Ectopic bone formation associated with recombinant human bone morphogenetic proteins-2 using absorbable collagen sponge and beta tricalcium phosphate as carriers. Biomaterials. 2005. 26:2501–2507.

8. Jung RE, Glauser R, Scharer P, Hammerle CH, Sailer HF, Weber FE. Effect of rhBMP-2 on guided bone regeneration in humans. Clin Oral Implants Res. 2003. 14:556–568.

9. Smeets R, Maciejewski O, Gerressen M, Spiekermann H, Hanisch O, Riediger D, et al. Impact of rhBMP-2 on regeneration of buccal alveolar defects during the osseointegration of transgingival inserted implants. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009. 108:e3–e12.

10. Wikesjo UM, Sorensen RG, Wozney JM. Augmentation of alveolar bone and dental implant osseointegration: clinical implications of studies with rhBMP-2. J Bone Joint Surg Am. 2001. 83:Suppl 1. (Pt 2):S136–S145.
11. Nevins M, Kirker-Head C, Nevins M, Wozney JA, Palmer R, Graham D. Bone formation in the goat maxillary sinus induced by absorbable collagen sponge implants impregnated with recombinant human bone morphogenetic protein-2. Int J Periodontics Restorative Dent. 1996. 16:8–19.
12. Lekholm U, Adell R, Lindhe J, Branemark PI, Eriksson B, Rockler B, et al. Marginal tissue reactions at osseointegrated titanium fixtures. (II) A cross-sectional retrospective study. Int J Oral Maxillofac Surg. 1986. 15:53–61.
13. Hall J, Sorensen RG, Wozney JM, Wikesjo UM. Bone formation at rhBMP-2-coated titanium implants in the rat ectopic model. J Clin Periodontol. 2007. 34:444–451.

14. Herr G, Hartwig CH, Boll C, Kusswetter W. Ectopic bone formation by composites of BMP and metal implants in rats. Acta Orthop Scand. 1996. 67:606–610.

15. Wikesjo UM, Qahash M, Polimeni G, Susin C, Shanaman RH, Rohrer MD, et al. Alveolar ridge augmentation using implants coated with recombinant human bone morphogenetic protein-2: histologic observations. J Clin Periodontol. 2008. 35:1001–1010.

16. Lee J, Decker JF, Polimeni G, Cortella CA, Rohrer MD, Wozney JM, et al. Evaluation of implants coated with rhBMP-2 using two different coating strategies: a critical-size supraalveolar peri-implant defect study in dogs. J Clin Periodontol. 2010. 37:582–590.

17. Susin C, Qahash M, Polimeni G, Lu PH, Prasad HS, Rohrer MD, et al. Alveolar ridge augmentation using implants coated with recombinant human bone morphogenetic protein-7 (rhBMP-7/rhOP-1): histological observations. J Clin Periodontol. 2010. 37:574–581.

18. Wikesjo UM, Susin C, Qahash M, Polimeni G, Leknes KN, Shanaman RH, et al. The critical-size supraalveolar peri-implant defect model: characteristics and use. J Clin Periodontol. 2006. 33:846–854.

19. Tatakis DN, Koh A, Jin L, Wozney JM, Rohrer MD, Wikesjo UM. Peri-implant bone regeneration using recombinant human bone morphogenetic protein-2 in a canine model: a dose-response study. J Periodontal Res. 2002. 37:93–100.

20. Decker JF, Lee J, Cortella CA, Polimeni G, Rohrer MD, Wozney JM, et al. Evaluation of implants coated with recombinant human bone morphogenetic protein-2 and vacuum-dried using the critical-size supraalveolar peri-implant defect model in dogs. J Periodontol. 2010. 81:1839–1849.

21. Becker J, Kirsch A, Schwarz F, Chatzinikolaidou M, Rothamel D, Lekovic V, et al. Bone apposition to titanium implants biocoated with recombinant human bone morphogenetic protein-2 (rhBMP-2). A pilot study in dogs. Clin Oral Investig. 2006. 10:217–224.

22. Jones AA, Buser D, Schenk R, Wozney J, Cochran DL. The effect of rhBMP-2 around endosseous implants with and without membranes in the canine model. J Periodontol. 2006. 77:1184–1193.

24. Park JC, So SS, Jung IH, Yun JH, Choi SH, Cho KS, et al. Induction of bone formation by Escherichia coli-expressed recombinant human bone morphogenetic protein-2 using block-type macroporous biphasic calcium phosphate in orthotopic and ectopic rat models. J Periodontal Res. 2011. 46:682–690.

25. Rohrer MD, Schubert CC. The cutting-grinding technique for histologic preparation of undecalcified bone and bone-anchored implants. Improvements in instrumentation and procedures. Oral Surg Oral Med Oral Pathol. 1992. 74:73–78.

26. Jeon O, Rhie JW, Kwon IK, Kim JH, Kim BS, Lee SH. In vivo bone formation following transplantation of human adipose-derived stromal cells that are not differentiated osteogenically. Tissue Eng Part A. 2008. 14:1285–1294.

27. Lee YJ, Jung SW, Chae GJ, Cho KS, Kim CS. The effect of recombinant human bone morphogenetic protein-2/macroporous biphasic calcium phosphate block system on bone formation in rat calvarial defects. J Korean Acad Periodontol. 2007. 37:Suppl. 397–407.

28. Lee JH, Kim CS, Choi KH, Jung UW, Yun JH, Choi SH, et al. The induction of bone formation in rat calvarial defects and subcutaneous tissues by recombinant human BMP-2, produced in Escherichia coli. Biomaterials. 2010. 31:3512–3519.

29. Bessho K, Konishi Y, Kaihara S, Fujimura K, Okubo Y, Iizuka T. Bone induction by Escherichia coli-derived recombinant human bone morphogenetic protein-2 compared with Chinese hamster ovary cell-derived recombinant human bone morphogenetic protein-2. Br J Oral Maxillofac Surg. 2000. 38:645–649.

30. Haidar ZS, Hamdy RC, Tabrizian M. Delivery of recombinant bone morphogenetic proteins for bone regeneration and repair. Part A: current challenges in BMP delivery. Biotechnol Lett. 2009. 31:1817–1824.

31. Seeherman H, Wozney JM. Delivery of bone morphogenetic proteins for orthopedic tissue regeneration. Cytokine Growth Factor Rev. 2005. 16:329–345.

32. Kawakatsu N, Oda S, Kinoshita A, Kikuchi S, Tsuchioka H, Akizuki T, et al. Effect of rhBMP-2 with PLGA/gelatin sponge type (PGS) carrier on alveolar ridge augmentation in dogs. J Oral Rehabil. 2008. 35:647–655.

33. Schliephake H, Weich HA, Dullin C, Gruber R, Frahse S. Mandibular bone repair by implantation of rhBMP-2 in a slow release carrier of polylactic acid: an experimental study in rats. Biomaterials. 2008. 29:103–110.

34. Le Nihouannen D, Saffarzadeh A, Gauthier O, Moreau F, Pilet P, Spaethe R, et al. Bone tissue formation in sheep muscles induced by a biphasic calcium phosphate ceramic and fibrin glue composite. J Mater Sci Mater Med. 2008. 19:667–675.

35. Le Nihouannen D, Guehennec LL, Rouillon T, Pilet P, Bilban M, Layrolle P, et al. Micro-architecture of calcium phosphate granules and fibrin glue composites for bone tissue engineering. Biomaterials. 2006. 27:2716–2722.

36. Hong SJ, Kim CS, Han DK, Cho IH, Jung UW, Choi SH, et al. The effect of a fibrin-fibronectin/beta-tricalcium phosphate/recombinant human bone morphogenetic protein-2 system on bone formation in rat calvarial defects. Biomaterials. 2006. 27:3810–3816.

37. Abarrategi A, Moreno-Vicente C, Ramos V, Aranaz I, Sanz Casado JV, Lopez-Lacomba JL. Improvement of porous beta-TCP scaffolds with rhBMP-2 chitosan carrier film for bone tissue application. Tissue Eng Part A. 2008. 14:1305–1319.

38. Alam I, Asahina I, Ohmamiuda K, Enomoto S. Comparative study of biphasic calcium phosphate ceramics impregnated with rhBMP-2 as bone substitutes. J Biomed Mater Res. 2001. 54:129–138.

39. Oda S, Kinoshita A, Higuchi T, Shizuya T, Ishikawa I. Ectopic bone formation by biphasic calcium phosphate (BCP) combined with recombinant human bone morphogenetic protein-2 (rhBMP-2). J Med Dent Sci. 1997. 44:53–62.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download