Abstract
Purpose
The aim of this study was to examine whether a previous peri-implantitis site can affect osseointegration, by comparing implant placement at a site where peri-implantitis was present and at a normal bone site. A second aim of this study was to identify the tissue and bone reaction after treating the contaminated implant surface to determine the optimal treatment for peri-implant diseases.
Methods
A peri-implant mucositis model for dogs was prepared to determine the optimal treatment option for peri-implant mucositis or peri-implantitis. The implants were inserted partially to a length of 6 mm. The upper 4 mm part of the dental implants was exposed to the oral environment. Simple exposure for 2 weeks contaminated the implant surface. After 2 weeks, the implants were divided into three groups: untreated, swabbed with saline, and swabbed with H2O2. Three implants from each group were placed to the full length in the same spot. The other three implants were placed fully into newly prepared bone. After eight weeks of healing, the animals were sacrificed. Ground sections, representing the mid-buccal-lingual plane, were prepared for histological analysis. The analysis was evaluated clinically and histometrically.
Results
The untreated implants and H2O2-swabbed implants showed gingival inflammation. Only the saline-swabbed implant group showed re-osseointegration and no gingival inflammation. There was no difference in regeneration height or bone-to-implant contact between in situ implant placement and implant placement in the new bone site.
Implant therapy is considered a predictable and safe treatment option for replacing missing teeth and is now believed to be a principal treatment procedure with a high success rate, irrespective of the prosthetic type [1]. The implant survival rate is now between 92% and 98% [2]. On the other hand, despite the high success rate, implants are still susceptible to peri-implant infections, i.e., peri-implant mucositis and peri-implantitis [3]. Peri-implant disease is defined as peri-implant mucosits and peri-implantitis [4]. Peri-implant mucositis is an inflammatory lesion with redness and swelling of the soft tissue that only resides in the mucosa, whereas peri-implantitis is often associated with suppuration and deepened pockets, and is always accompanied by the loss of supporting bone [5,6]. With an increasing number of implant patients, the rate of peri-implant diseases has increased at an incidence ranging from 0.5 to 3% per year [7]. Zitzmann and Berglundh [5] examined the prevalence of peri-implant disease. Peri-implant mucositis occurred in 80% of subjects and in 50% of implant sites. Peri-implantitis was identified in 28% of subjects and 43% of implant sites.
There is considerable evidence suggesting that the etiology of peri-implant diseases is of a microbial nature [4,8]. As peri-implantitis is also classified as a disease process associated with the microorganisms from chronic periodontitis [4,8,9], it is assumed that the removal of these bacterial plaque biofilms from the implant surface should be essential to stop the progression of the disease [10].
A range of therapies has been suggested for the treatment of peri-implant diseases in animals [10-18]. Air-powder abrasive units, citric acid, chlorhexidine irrigation, carbon dioxide lasers, rotating brushes with pumice, or cotton pellets soaked in saline, hydrogen peroxide, or chlorhexidine have been used alone or in various combinations. On the other hand, the peri-implantitis model using ligature wires has shown extensive uncontrollable bone destruction. Controlling the multiple factors affecting the healing process, such as wound stability, clot adhesion and cellular migration, is difficult [17,19]. Kolonidis et al. [19] suggested a new peri-implantitis model to examine surface decontamination and re-osseointegration. In this model, a machined surface exposed for 5 weeks allowed surface contamination to occur. The contaminated surface was then cleaned and treated. After the treatment, re-osseointegration has been achieved. The other concern is the effect of inflammatory tissues in re-osseointegration. Schou et al. [20] compared the inflammatory response and the loss of supporting bone in ligature-induced peri-implantitis and ligature-induced periodontitis. They found a large amount of bone loss and inflammatory infiltration around the implants and ankylosed teeth. They concluded that the absence of a periodontal ligament might have a lesion-promoting effect on the pathogenesis of peri-implantitis. Another study about the biology of peri-implantitis, after persistent biofilm accumulation, showed that inflammatory infiltration in the implanto- mucosal unit was almost three times greater than in the dento-gingival unit [21]. Implant placement at the place of peri-implantitis may disturb re-osseointegration and the healing of supporting tissues.
Therefore, this study examined whether a previous peri-implantitis site can affect osseointegration, by comparing implant placement at the site where peri-implantitis was present and at the normal bone site. The other aim of this study was to identify the tissue and bone reaction after treating the contaminated implant surface to determine the optimal treatment for peri-implant diseases.
Two mongrel dogs (≥30 kg) were prepared for implant installation. Animal selection and management, surgical protocol, and preparation followed the protocols approved by the Institutional Animal Care and Use Committee, Yonsei Medical Center, Seoul, Korea (IRB No. 09-067).
The dental implants were sandblasted with a large grit and acid-etched (SLA) with 3.8 mm diameter, 10 mm length (Dentium, Seoul, Korea).
All surgical procedures including the extraction and experiment were performed under general anesthesia. Induction by an intravenous injection of atropin and an intramuscular injection of a combination of xylazine and ketamin were performed and general anesthesia was maintained with inhalation anesthesia.
The premolars (P1 to P4) of the dogs were extracted. After extracting the premolars, an 8-week healing period was allowed for complete healing of the extraction sockets.
After 8 weeks, general anesthesia was administered again and a full thickness flap was reflected from each edentulous region.
Six dental implants were partially inserted (6 mm) in both sides of the mandible (Fig. 1). This resulted in 4 mm of the implant being exposed above the bone. Two implants were placed at one side, and the other four implants were placed at the other side of the mandible (Fig. 2). A total of six implants were partially inserted.
The flap was repositioned and sutured with 4-0 monosyn (B. Braun Melsungen AG, Melsungen, Germany). The sutures were removed one week after surgery. Two weeks were given for healing and plaque accumulation, to induce surface contamination (Fig. 3). After administering general anesthesia again, a full thickness flap was reflected, as described earlier.
The implants with contaminated surfaces were divided into two groups and two implants of each group were treated with different cleansing techniques, while the remaining one was left as the control group. The two different cleaning techniques were 1) swabbing with saline and, 2) swabbing with 10% hydrogen peroxide (H2O2). After cleaning the implant surface, the implants were re-inserted in full length. In one group, the three implants were placed on the very spot to the full length after full length drilling. The other three implants in the other group were placed fully in newly prepared bone (Figs. 4 and 5). The flap was repositioned and sutured with 4-0 monosyn (B. Braun Melsungen AG). The sutures were removed one week after surgery. After eight weeks of healing, the animals were sacrificed.
The ground sections, representing the mid-buccal-lingual plane, were prepared for the histology analysis. The sections were stained with Goldner trichrome stain. The histological slides were magnified 12.5 times under optical microscopy and the images were captured.
The analysis was performed clinically and histometrically. Linear measurements of the bone regeneration height were taken at both the buccal and lingual sides. The percentage of bone to implant contact (BIC) was measured at the pre-contaminated site. The two histometric measurements were performed with Image-pro (MediaCybernetics Inc., Bethesda, MD, USA).
The untreated implants showed less osseointegration in the pre-contaminated site and showed no osseointegration in the newly prepared site. The H2O2 swabbing groups showed less osseointegration in both the pre-contaminated site and the newly prepared site. On the other hand, the saline swabbing groups showed more osseointegration than the other groups. No remarkable difference in the pre-contaminated site and newly prepared site was visible.
The implants were not treated, and were placed in a pre-contaminated site (Fig. 6). No bone regeneration or connective tissue attachment was observed around the contaminated implant surface area.
The implants were not treated, and were placed in a newly prepared normal bone site (Fig. 7). No bone regeneration or connective tissue attachment was observed.
The implants were swabbed with saline and placed in the pre-contaminated site (Fig. 8). The contaminated implants swabbed with saline showed bone regeneration and connective tissue attachment around the implants.
The implants were swabbed with saline, and placed in the newly prepared normal bone site (Fig. 9). Osseointegration with bone regeneration and soft tissue attachment was observed.
The implants were swabbed with H2O2, and placed in a pre-contaminated site (Fig. 10). Inflammation was observed around the implants. Some soft tissue attachment was noted. Less osseointegration was observed compared to the saline swabbing group.
The implants were swabbed with H2O2, and placed in a newly prepared normal bone site (Fig. 11). Contaminated implants that had been inserted in the newly prepared site showed inflammation around the implants and less osseointegration.
Tables 1 and 2 list the results of the morphometric measurements. The regenerated bone heights in the saline groups were 3.12 mm in the in situ group and 3.44 mm in the new bone site group, whereas the control group showed less or no bone regeneration (1.16 mm and 0 mm) and the H2O2 group showed less bone regeneration (1.68 mm and 1.27 mm) than the saline groups (Table 1). The bone to implant contact showed similar results to the bone regeneration height except for the saline group in the newly prepared bone (Table 2). Both treatment modalities in the two groups showed high BICs around the implants, whereas the control group showed less or no BIC. BICs in the control group were 8.7% and 0%. Treatment with saline showed the highest bone regeneration and BIC compared to the untreated and H2O2 groups.
Although several studies have shown that re-osseointegration in such areas is difficult to obtain [16,22], these results show that osseointegration could occur on a surface previously contaminated with dental plaque. The results are consistent with many studies [19,23-25].
The experimental models inducing peri-implantitis have been examined. The standard peri-implantitis model was employed as the defect model using ligature wires [10,14-16,23]. However, the ligature-induced peri-implantitis model had limitations in that the elements of clot adhesion, wound stability and cellular migration/differentiation patterns could affect the outcome in addition to any proposed effect of the surface contamination [19]. In the present study, the implants were inserted partially to a length of 6 mm. The upper 4 mm part of the dental implants was exposed to the oral environment. Simple exposure for 2 weeks contaminated the implant surface. The control group showed no osseointegration after 8 weeks of healing. This demonstrates that the contamination of the implant surface in this model is sufficient to induce peri-implantitis.
The implant surface characteristics can affect the treatment outcome of peri-implantitis. According to a widely held opinion, re-osseointegration is difficult to obtain [22,26]. On the other hand, Albouy et al. [27] examined the effect of surgical treatment of peri-implantitis without systemic antibiotics for the four types of implants. They concluded that the resolution of peri-implantitis after treatment without systemic or local antimicrobial therapy is possible with surgical treatment in SLA surface implants. It is anticipated that the SLA surface which was also examined in this study might provide good stability for the coagulum that forms in the defect region after surgery [16].
In the present study, treatment modalities, such as saline and H2O2 swabbing, seemed to be sufficient to treat the decontaminated surfaces. These findings are in agreement with those reported by Kolonidis et al. [19]. The therapies proposed for the treatment of peri-implant diseases are based on the evidence available from the treatment of periodontitis [21,28]. As both periodontitis and peri-implantitis are opportunistic infections, their treatment must be anti-infective and the same clinical principles must be applied. On the other hand, due to surface characteristics, there have been technical difficulties in the treatment of contaminated dental implant surfaces. It was assumed that the removal of bacterial plaque from the implant surface might be essential to achieve re-osseointegration [10]. Two different treatment modalities were suggested, cleaning with saline and H2O2. The BIC in both groups were slightly lower than previous studies. Persson et al. [16] reported that the BIC in old bone was 70.7% in SLA dental implants. With the exception of the saline in situ group (65.4%), they showed low BICs. Both groups showed better results than the control group. In the bone regeneration height, the saline group showed better results than the control and H2O2 groups. It is assumed that cleaning with saline can be a treatment modality for the decontamination of peri-implantitis in implant surfaces. This result is in agreement with previous studies [13,15,19].
This study also examined whether a previously peri-implant diseased site can affect osseointegration. This was achieved by placing the contaminated implants at a previously peri-implant diseased site and normal edentulous bone site. The hypothesis was that the characteristics of peri-implantitis affect the infiltration of inflammation direct to the bone, which disturbs re-osseointegration. The results showed no difference in regeneration height or BIC between in situ implant placement and implant placement in the new bone site except BIC in the saline group. The saline group in the newly prepared bone showed little BIC compared to the regenerated bone height, which was assumed to be caused by the histological defects around the implants (Fig. 9) and the small number of samples. Therefore, it could be concluded that implant installation in a previously peri-implant diseased site might not interfere with osseointegration. Clinically, if there is no problem in soft tissue management, immediate implantation after removing a failed implant could be possible.
This investigation had some limitations. This study was a pilot study and a small number of animals were used. A large sample and more treatment modalities should be assessed to study the treatment options for peri-implant diseases.
Re-osseointegration can occur partially in a cleansed implant surface that has been contaminated with a dental plaque biofilm. Cleaning with saline may be effective in managing implant decontamination. After implant surface decontamination, implant installation in a previously peri-implant diseased site may not interfere with osseointegration.
Figures and Tables
Figure 3
Clinical photograph illustrating the peri-implant inflammation around partially installed implants. Arrows: gingival swelling with inflammatory tissue around implants.
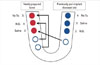
Figure 4
Schematic drawing describing the full length implant installation after treatment. Implants in one side were re-installed in situ (blue circles). Implants in the other side were re-installed in the newly prepared bone site (red circles). Tx.: treatment.
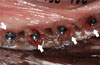
Figure 5
Clinical photographs illustrating the re-installed implants after decontamination. Implants after each treatments were inserted in the edentulous normal bone site (A) and in the previously peri-implant diseased site (B).
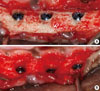
Figure 6
Histological presentation of a specimen of the control group installed in situ. Note that the bone to implant contacts are limited below the defect margin. White line: defect margin, Arrows: regenerated bone height (Goldner trichrome staining, ×25).

Figure 7
Histological presentation of a specimen of the control group installed in an edentulous normal bone site. No bone regeneration was observed above the defect margin. Line: defect margin (Goldner trichrome staining, ×25).

Figure 8
Histological presentation of a specimen of the group treated with saline, and installed in situ. Note the pre-contaminated surface covered with newly regenerated bone. Line: defect margin, Arrows: regenerated bone height (Goldner trichrome staining, ×25).

Figure 9
Histological presentation of a specimen of the group treated with saline, and installed in an edentulous normal bone site. Line: defect margin, Arrows: regenerated bone height (Goldner trichrome staining, ×25).

Figure 10
Histological presentation of a specimen of the group treated with H2O2, and installed in situ. Note the pre-contaminated part of the implant surface, which was not fully covered. Line: defect margin, Arrows: regenerated bone height (Goldner trichrome staining, ×25).

ACKNOWLEDGEMENTS
This study was supported by a faculty research grant of Yonsei University College of Dentistry for 2010 (6-2010-0099).
References
1. Wennerberg A, Albrektsson T. Current challenges in successful rehabilitation with oral implants. J Oral Rehabil. 2011. 38:286–294.

2. Lambert FE, Weber HP, Susarla SM, Belser UC, Gallucci GO. Descriptive analysis of implant and prosthodontic survival rates with fixed implant-supported rehabilitations in the edentulous maxilla. J Periodontol. 2009. 80:1220–1230.

3. Renvert S, Persson GR. Periodontitis as a potential risk factor for peri-implantitis. J Clin Periodontol. 2009. 36:Suppl 10. 9–14.

4. Mombelli A, van Oosten MA, Schurch E Jr, Land NP. The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiol Immunol. 1987. 2:145–151.

5. Zitzmann NU, Berglundh T. Definition and prevalence of peri-implant diseases. J Clin Periodontol. 2008. 35:8 Suppl. 286–291.

6. Lindhe J, Meyle J. Group D of European Workshop on Periodontology. Peri-implant diseases: Consensus Report of the Sixth European Workshop on Periodontology. J Clin Periodontol. 2008. 35:8 Suppl. 282–285.

7. Berglundh T, Persson L, Klinge B. A systematic review of the incidence of biological and technical complications in implant dentistry reported in prospective longitudinal studies of at least 5 years. J Clin Periodontol. 2002. 29:Suppl 3. 197–212.

8. Alcoforado GA, Rams TE, Feik D, Slots J. Microbial aspects of failing osseointegrated dental implants in humans. J Parodontol. 1991. 10:11–18.

9. Leonhardt A, Renvert S, Dahlén G. Microbial findings at failing implants. Clin Oral Implants Res. 1999. 10:339–345.

10. Schwarz F, Jepsen S, Herten M, Sager M, Rothamel D, Becker J. Influence of different treatment approaches on non-submerged and submerged healing of ligature induced peri-implantitis lesions: an experimental study in dogs. J Clin Periodontol. 2006. 33:584–595.

11. Schwarz F, Bieling K, Latz T, Nuesry E, Becker J. Healing of intrabony peri-implantitis defects following application of a nanocrystalline hydroxyapatite (Ostim) or a bovine-derived xenograft (Bio-Oss) in combination with a collagen membrane (Bio-Gide). A case series. J Clin Periodontol. 2006. 33:491–499.

12. Sculean A, Schwarz F, Becker J. Anti-infective therapy with an Er:YAG laser: influence on peri-implant healing. Expert Rev Med Devices. 2005. 2:267–276.

13. Schou S, Holmstrup P, Skovgaard LT, Stoltze K, Hjørting-Hansen E, Gundersen HJ. Autogenous bone graft and ePTFE membrane in the treatment of peri-implantitis. II. Stereologic and histologic observations in cynomolgus monkeys. Clin Oral Implants Res. 2003. 14:404–411.

14. Schou S, Holmstrup P, Jørgensen T, Skovgaard LT, Stoltze K, Hjørting-Hansen E, et al. Implant surface preparation in the surgical treatment of experimental peri-implantitis with autogenous bone graft and ePTFE membrane in cynomolgus monkeys. Clin Oral Implants Res. 2003. 14:412–422.

15. Persson LG, Araújo MG, Berglundh T, Gröndahl K, Lindhe J. Resolution of peri-implantitis following treatment. An experimental study in the dog. Clin Oral Implants Res. 1999. 10:195–203.
16. Persson LG, Berglundh T, Lindhe J, Sennerby L. Re-osseointegration after treatment of peri-implantitis at different implant surfaces. An experimental study in the dog. Clin Oral Implants Res. 2001. 12:595–603.
17. Alhag M, Renvert S, Polyzois I, Claffey N. Re-osseointegration on rough implant surfaces previously coated with bacterial biofilm: an experimental study in the dog. Clin Oral Implants Res. 2008. 19:182–187.
18. Parlar A, Bosshardt DD, Cetiner D, Schafroth D, Unsal B, Haytaç C, et al. Effects of decontamination and implant surface characteristics on re-osseointegration following treatment of peri-implantitis. Clin Oral Implants Res. 2009. 20:391–399.
19. Kolonidis SG, Renvert S, Hämmerle CH, Lang NP, Harris D, Claffey N. Osseointegration on implant surfaces previously contaminated with plaque. An experimental study in the dog. Clin Oral Implants Res. 2003. 14:373–380.

20. Schou S, Holmstrup P, Reibel J, Juhl M, Hjørting-Hansen E, Kornman KS. Ligature-induced marginal inflammation around osseointegrated implants and ankylosed teeth: stereologic and histologic observations in cynomolgus monkeys (Macaca fascicularis). J Periodontol. 1993. 64:529–537.

21. Heitz-Mayfield LJ, Lang NP. Comparative biology of chronic and aggressive periodontitis vs. peri-implantitis. Periodontol 2000. 2010. 53:167–181.

22. Grunder U, Hürzeler MB, Schüpbach P, Strub JR. Treatment of ligature-induced peri-implantitis using guided tissue regeneration: a clinical and histologic study in the beagle dog. Int J Oral Maxillofac Implants. 1993. 8:282–293.

23. Jovanovic SA, Kenney EB, Carranza FA Jr, Donath K. The regenerative potential of plaque-induced peri-implant bone defects treated by a submerged membrane technique: an experimental study. Int J Oral Maxillofac Implants. 1993. 8:13–18.

24. Mohamed S, Polyzois I, Renvert S, Claffey N. Effect of surface contamination on osseointegration of dental implants surrounded by circumferential bone defects. Clin Oral Implants Res. 2010. 21:513–519.

25. Renvert S, Samuelsson E, Lindahl C, Persson GR. Mechanical non-surgical treatment of peri-implantitis: a double-blind randomized longitudinal clinical study. I: clinical results. J Clin Periodontol. 2009. 36:604–609.

26. Persson LG, Ericsson I, Berglundh T, Lindhe J. Osseintegration following treatment of peri-implantitis and replacement of implant components. An experimental study in the dog. J Clin Periodontol. 2001. 28:258–263.





 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download