Abstract
Purpose
The aim of this study was to investigate the effects of synthetic fibronectin (FN) fragments, including fibrin binding sites from amino-terminal FN fragments containing type I repeats 1 to 5, on osteoblast-like cell activity.
Methods
Oligopeptides ranging from 9 to 20 amino acids, designated FF1, FF3, and FF5, were synthesized by a solid-phase peptide synthesizing system, and we investigated the effects of these peptides on cell attachment and extent of mineralization using confocal microscopy, 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assays, and Alizarin red S staining.
Results
FF3 and FF5 peptides increased the number of attached human osteoblastic cells, and FF3 administration led to prominent cell spreading. Mineralization was increased in FF3 and FF5 compared to FF1 and the untreated control.
Conclusions
Taken together, it can be concluded that the fibrin-binding oligopeptides FF3 and FF5 enhanced cell attachment and mineralization on osteoblast-like cells. These results indicate that FF3 and FF5 have the potential to increase osteoblast-like cell activity. Performing an in vivo study may provide further possibilities for surface modification of biomimetic peptides to enhance osteogenesis, thus improving the regeneration of destroyed alveolar bone.
The structural and functional reconstruction of collapsed periodontal tissues caused by periodontitis is one of the major goals of periodontal treatment. The restoration of destroyed periodontal tissues may require regeneration of alveolar bone, cementum, periodontal ligaments, and other connective tissue cells. Complex interactions between cells and extracellular matrix (ECM) components in gingival connective tissue are crucial in this periodontal wound healing process [1,2].
One of the major components of ECM is fibronectin (FN), a large glycoprotein, which interacts with cells and transmits signals through numerous receptors [3]. FN mediates a wide variety of cellular interactions with ECM and plays important roles in cell adhesion, migration, proliferation, and differentiation [4]. Among bone ECM proteins, FN is present in its highest concentrations during osteogenesis and binds to osteoblasts more strongly than do other ECM proteins such as collagen type 1, laminin, or vitronectin [5]. Studies have shown that FN enhances the expression of several phenotypic markers of osteoblasts, including alkaline phosphatase activity, osteocalcin, and Osf2/Cbfa1, a key transcription factor in osteoblastic differentiation [6]. Majmudar et al. [7] observed that synthesis and deposition of FN, collagen type I, and collagen type III molecules occurred in chick embryo calvaria as well during osteoblast mineralization in vitro [7].
FN usually exists as a dimer composed of two nearly identical-250 kDa subunits linked covalently near their C-termini by two disulfide bonds. Each monomer consists of three types of repeating units: 12 type I repeats, 2 type II repeats and 15-17 type III repeats, which together account for approximately 90% of the FN sequence [8].
Within the structure of FN, an Arg-Gly-Asp (RGD) sequence in the tenth type III domain and a Pro-His-Ser-Arg-Asn (PHSRN) sequence in the ninth type III domain are involved in cell adhesion via binding with α5β1 integrin, which is the most common FN receptor as well as the mediator of signal transmission between FN and cells [9,10].
FN contains adhesion domains for many other molecules, including fibrin, heparin, and collagen. For example, a glutamine residue at the amino terminus serves as a transglutamination site for activated factor 13, thereby crosslinking FN to a variety of proteins, including fibrin and fibrinogen [11]. Following tissue injury, the formation of blood clots serves to restore vascular integrity as well as provide a provisional matrix for the initiation of wound repair. Fibrin and plasma FN are essential to these functions, as they are the major protein components of blood clots [12]. In blood clotting systems, fibrin deposition is followed by the covalent attachment of plasma FN to the fibrin matrix, which promotes fibroblast adhesion, spreading, and migration into the clot [13,14]. This migration is followed by the deposition of cellular FN, which serves as a temporary scaffold for further recruitment of cells and granulation tissue formation in wound healing processes [15]. Many studies have concluded that fibroblast transmigration into the fibrin matrix requires the presence of FN [16,17].
Each FN subunit contains two major fibrin-binding sites, amino-terminal type I repeats 1 to 5 and carboxy-terminal type I repeats 10 to 12, which mediate noncovalent interaction with fibrin. Covalent FN-fibrin binding activated by factor 13 involves glutamine residues localized to a 27 kDa amino-terminal FN fragment that contains type I repeats [18-20].
The aim of this study is to investigate the effects of synthetic FN fragments, including fibrin binding sites from amino-terminal FN fragments containing type I repeats 1 to 5, on osteoblast-like cell activity.
Without adverse reactions such as immunogenicity or matrix degradation [21], short biomimetic peptides containing specific sequences serve as good substitutes for native FN on osteogenesis [22,23]. Moreover, this study presents the possibility of using FN peptide-modified biomaterials to induce fibrin-related osteogenesis in vivo [24].
Oligopeptides were derived from the primary and tertiary human plasma FN structures. A solid-phase peptide synthesizing system was used to prepare these oligopeptides. Amino acid sequences of synthetic oligopeptides are as follows: FF1 (CYDNGKHYQ); FF3 (CFDKYTGNTYRVGDTYERPK); FF5 (CTSRNRCNDQ). These three fragments were designed using the amino terminal fibrin-binding sequence of human FN (FINC_HUMAN: P02751). The sequence of FF1 was derived from type I repeat 1; FF3 from type I repeat 2; and FF5 from type I repeats 4 to 5.
HOS cells were cultured in α-modified Eagle's medium (α-MEM; Gibco, Grand Island, NY, USA), supplemented with 10% (v/v) fetal bovine serum (FBS, Gibco) and 1% streptomycin at 37℃ in 5% CO2, and changed every 3 days. When the HOS cells reached confluence, the cell layer was rinsed with phosphate-buffered saline (PBS, Gibco) and released with 0.05% trypsin-ethylenediaminetetraacetic acid (EDTA) for 5 minutes. The cells were collected by 300×g centrifugation and resuspended in the culture medium. HOS cells between passages 8 and 11 were used in this study.
Synthetic oligopeptides FF1, 3, and 5 were diluted in PBS to 100 µM and filtered by syringe filter. A 4-well Lab-Tek II chamber slide (Nalge Nunc International, Naperville, IL, USA) was coated with 50 µL of 100 µM FF1, 3, and 5 and a 96-well chamber slide was coated with 10 µL of the same peptide solutions. The coated slides were dried overnight with an opened cover at 4℃ on the bench and then were blocked with 1% (w/v) bovine serum albumin (BSA) for 1 hour.
After rinsing to remove BSA, 24 hours serum starved HOS cells were plated at 3×104 cells/well in α-MEM with 0.1% BSA. To ensure sufficient adhesion, the cells were incubated in 37℃ 5% CO2 for 12 hours.
Adherent HOS cells in the 4-well chamber were fixed with 10% neutral buffered formalin (500 µL/well; Sigma-Aldrich Co., St. Louis, MO, USA) and stored at room temperature for 10 minutes. The fixed cells were washed twice with PBS (13 mMNaCl, 0.2 mM KC1, 0.8 mM Na2HP04, 0.2 mM KH2P04, pH 7.4), then treated with 0.1% Triton-X (Sigma Aldrich Co.) for 10 minutes to widen the cell membrane. The cells were then incubated with 1% BSA for 10 minutes, and the cells were stained with Hoechst 33342 (5 µL/well; Pierce Biotechnology, Rockford, IL, USA) and Alexa Flour 546-Phalloidin (5 µL/well) with PBS (300 µL/well) in the dark for 20 minutes at room temperature. Washing with PBS 2 to 3 times was performed in each step. After staining the nuclei and actin microfilaments, mounting solution (Dako, Glostrup, Denmark) was added and the cells were stored for 4 hours. The fluorescent images were digitally captured with a laser scanning microscope (LSM-GB200, Olympus Co., Tokyo, Japan).
For adherent HOS cells in the 96-well chamber, MTT solution (Promega Co., Madison, WI, USA), which measures viable cells, was added (50 µL per well at 2 mg/mL) as described by the manufacturer. After 4 hours, all solutions were removed and the same volume of dimethyl sulfoxide was added followed by vortexing. The absorbance was measured at 540 nm.
For measurement of mineralization activity, HOS cells were cultured for 10 days in osteogenic induction medium according to the protocol described by Jaiswal et al. [25]. The cells were detached by trypsin-EDTA, resuspended in media (α-MEM) supplemented with 10% (v/v) FBS and 1% streptomycin, and 3×103 cells per well were seeded into a 24-well plate (non-tissue culture plate) and 4-well Lab-Tek II chamber slide (Nalge Nunc International). During the 10 days of mineralization, osteogenic induction medium (100 nM dexamethasone, 1M β-glycerophosphate [Sigma-Aldrich Co.], 0.05 mM L-ascorbic acid-2-phosphate [Fluka Biochemika, Buchs, Switzerland], and 10% FBS in α-MEM with streptomycin) was changed every 3 days while supplementing with fresh 100 µM peptide FF1, 3, or 5 solution (100 µL/well) [26]. Mineralization was evaluated by confocal microscopy for calcium deposition and also by Alizarin red S staining.
Alizarin red S (Sigma-Aldrich Co.) staining in the 24-well plates was performed after aspirating the medium from each well so as not to disturb the cells. The cells were incubated in ice-cold 95% ethanol for 15 minutes at room temperature before carefully aspirating the alcohol and rinsing twice (5 minutes each) with double distilled water. The water was aspirated and 1 mL of 2% Alizarin red S solution was added. The plate was incubated at room temperature for 5 minutes before removing the Alizarin red S solution and the wells were washed five times with 2 mL distilled water. The pH value of the Alizarin red S solution was adjusted to 4.1 to 4.3 with ammonium hydroxide prior to the procedure.
Calcein (10 µL/40 ulmedia; Wako, Richmond, VA, USA) was injected into each well every 3 days, concurrent with adding fresh osteogenesis induction medium. After 10 days of mineralization, the cells were stained with Hoechst 33342 (5 µL/well; ICN Pharmaceuticals Inc., CA, USA), Alexa Flour 546-Phalloidin (5 µL/well), and calcein. These fluorescent dyes stain nuclei, actin filaments, and calcium, respectively, resulting in labeling of all actively mineralizing surfaces. Mounted 4-well specimens were characterized using a laser scanning microscope (LSM-GB200, Olympus Co.).
HOS cells were used as a model to investigate osteoblast activity due to their similarity to normal human osteoblasts in differentiated cell function, integrin expression, and adhesion patterns [27].
The addition of three synthetic fragments of FN corresponding to amino terminal fibrin-binding sequences FF1, 3, and 5 promoted the attachment and spreading of HOS cells compared with the untreated control (Figs. 1 and 2). Through confocal microscopy, blue-stained nuclei and red actin cytoskeletons were clearly labeled. After 12 hours of incubation, HOS cells incubated with FF3 and FF5 displayed an augmented number of attached cells, whereas FF1 only affected cell adhesion slightly. In the FF3-treated wells, adherent HOS cells exhibited remarkable spreading shapes with filopodia-like extensions when compared with the FF5-treated cells. In the FF5-treated wells, numerous HOS cells were rounded, but the difference was small. In the FF1-coated wells, attached cells appeared to extend much more than control cells, despite the limited number of adhered cells.
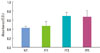
The MTT assay data correlated with peptide cell adhesion results. Absorbance measurements of formazan produced by HOS cells in the FF1-, 3-, and 5-coated wells is depicted in Fig. 2. The mean values of the repeated experiments differed among the no-treatment (NT), FF1, FF3, and FF5 samples. FF3- and FF5-coated wells resulted in better attachment compared with FF1-coated wells; however, this difference was not statistically significant (P=0.17). The unmodified wells served as a control.
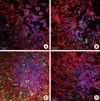
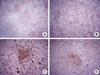
Mineralized specimens that incorporated calcein for 10 days were labeled green, blue, and red in confocal laser scanning microscopy. HOS cells densely proliferated, especially in the FF3- and FF5-injected cells (Fig. 3). Compared to FF5, FF3-injected cells showed higher levels of spread, and displayed increased green staining indicative of calcium levels. FF1-injected cells displayed a proliferation density similar to control cells, but the ability to spread was much greater than in the controls. Alizarin red S staining depicts how the extent of mineralization was quite different among the FF3, 5 and FF1, and NT groups (Fig. 4).
Biomimetic approaches to studying FN-induced physiological changes have often demonstrated increases in cellular activity. Specifically, the ninth and tenth FN type III repeats, which contain RGD and PHSRN cell attachment sequences, support maximal fibroblast attachment, but not migration of primary dental fibroblasts. Specific sequences like heparin domains are required for migration [28]. In addition, there have been many studies regarding HOS cell proliferation influenced by the ninth and tenth FN type III repeats because of binding specificity on integrins.
In the present study, we examined the adhesion, proliferation, and mineralization of HOS cells on FN mimetic peptides that contain fibrin binding sites. HOS cells were chosen for the current study because they are similar to osteoblasts in many ways, including their rapid proliferation [29,30]. The amino acid fragments used in this study were obtained from the NH2-terminal fibrin-binding site on human FN [31]. The fibrin-binding region contains type I repeat modules, which are maintained in the tertiary structure by fixed and intimate hydrophobic interactions with a non-conserved tryptophan residue in the fourth type I module. Specific binding to fibrin of these regions has been observed in many studies [32]. Fragments of FN containing the amino terminus have resulted in enhanced macrophage fibrin binding to the same extent as intact FN. This region contains a critical glutaminyl residue (Gln) that is the site of plasma transglutaminase (factor X IIIa)-mediated covalent cross-linking of FN to a lysyl residue located in the fibrin α-chain [33]. However, until this study, the influence of this fibrin-binding site on osteoblast cells had not been established.
In this study, significantly increased cellular attachment and mineralization were observed when HOS cells were exposed to fibrin-binding fragments (type I 2 to 5 repeats) FF3 (CFDKYTGNTYRVGDTYERPK) and FF5 (CTSRNRCNDQ). In addition, inactive cells incubated with the type I 1 repeat FF1 (CYDNGKHYQ) also resulted in increased spreading when compared to the untreated control. Using an MTT assay to measure attachment, we found that FF3- and FF5-exposed cells displayed different attachment levels than those exposed to FF1. Although cells aggregated to high levels in the 10 day mineralization assay, the FF3- and FF5-incubated cells showed a noticeable difference from the control cells by calcein staining and Alizarin red S staining. Therefore, FF3 and FF5 may be applicable for use in dental materials to improve osteogenesis via the recruitment of osteoblastic cells in the blood clotting process, thus improving the regeneration of alveolar bone.
According to previous studies, a Gln residue in the amino terminus of the FN protein is a significant factor in crosslinking to various other proteins, including fibrin and fibrinogen [34]. However, in this study, we observed that small segmented oligopeptides that do not contain this Gln residue (FF3) are sufficient to facilitate heightened osteogenic maturation of undifferentiated osteosarcoma cells, especially when compared to peptides that do contain this residue (FF1 and FF5).
To our knowledge, there are no published studies of the effects of the fibrin-binding domain of human FN on osteogenesis. The biological effect of fibrin-binding oligopeptides in this study supports the theory that FN plays a decisive role in bone regeneration as a temporary scaffold in the FN-fibrin matrix. Further studies are needed to apply these synthetic peptides to practical use, such as other in vitro models that add exogenous human fibrinogen, thrombin, and coagulation factor to form FN-fibrin clots.
Fibrin-binding oligopeptides denoted that FF3 and FF5 enhanced the cell attachment and mineralization of osteoblast-like cells. These results indicate that FF3 and FF5 have the potential to increase osteoblast-like cell activity. Therefore, these peptides may be feasible for surface modification of biomaterials to enhance osteogenesis and may thus improve the physiological regeneration of destroyed alveolar bone.
Figures and Tables
Figure 1
Humanosteoblastic cell attachment using confocal laser scanning microscopy. Nuclei are blue, and actin cytoskeletons are red. (A) Not treated (NT), (B) FF1, (C) FF3, (D) FF5.
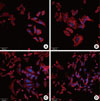
Figure 2
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay of humanosteoblastic cell attachment after 12 hours. NT: not treated.
References
1. Damsky CH. Extracellular matrix-integrin interactions in osteoblast function and tissue remodeling. Bone. 1999. 25:95–96.

2. Embery G, Waddington RJ, Hall RC, Last KS. Connective tissue elements as diagnostic aids in periodontology. Periodontol 2000. 2000. 24:193–214.

3. Rosso F, Giordano A, Barbarisi M, Barbarisi A. From cell-ECM interactions to tissue engineering. J Cell Physiol. 2004. 199:174–180.

4. Mosher DF. Fibronectin. 1989. San Diego: Academic Press.
5. Garcia AJ, Ducheyne P, Boettiger D. Effect of surface reaction stage on fibronectin-mediated adhesion of osteoblast-like cells to bioactive glass. J Biomed Mater Res. 1998. 40:48–56.

6. Ding HT, Wang CG, Zhang TL, Wang K. Fibronectin enhances in vitro vascular calcification by promoting osteoblastic differentiation of vascular smooth muscle cells via ERK pathway. J Cell Biochem. 2006. 99:1343–1352.

7. Majmudar G, Bole D, Goldstein SA, Bonadio J. Bone cell culture in a three-dimensional polymer bead stabilizes the differentiated phenotype and provides evidence that osteoblastic cells synthesize type III collagen and fibronectin. J Bone Miner Res. 1991. 6:869–881.

9. Mardon HJ, Grant KE. The role of the ninth and tenth type III domains of human fibronectin in cell adhesion. FEBS Lett. 1994. 340:197–201.

10. Redick SD, Settles DL, Briscoe G, Erickson HP. Defining fibronectin's cell adhesion synergy site by site-directed mutagenesis. J Cell Biol. 2000. 149:521–527.

11. Magnusson MK, Mosher DF. Fibronectin: structure, assembly, and cardiovascular implications. Arterioscler Thromb Vasc Biol. 1998. 18:1363–1370.
12. Corbett SA, Lee L, Wilson CL, Schwarzbauer JE. Covalent cross-linking of fibronectin to fibrin is required for maximal cell adhesion to a fibronectin-fibrin matrix. J Biol Chem. 1997. 272:24999–25005.

13. Grinnell F, Feld M, Minter D. Fibroblast adhesion to fibrinogen and fibrin substrata: requirement for cold-insoluble globulin (plasma fibronectin). Cell. 1980. 19:517–525.

14. Corbett SA, Wilson CL, Schwarzbauer JE. Changes in cell spreading and cytoskeletal organization are induced by adhesion to a fibronectin-fibrin matrix. Blood. 1996. 88:158–166.

15. Greiling D, Clark RA. Fibronectin provides a conduit for fibroblast transmigration from collagenous stroma into fibrin clot provisional matrix. J Cell Sci. 1997. 110(Pt 7):861–870.

16. Clark RA, Lin F, Greiling D, An J, Couchman JR. Fibroblast invasive migration into fibronectin/fibrin gels requires a previously uncharacterized dermatan sulfate-CD44 proteoglycan. J Invest Dermatol. 2004. 122:266–277.

17. Knox P, Crooks S, Rimmer CS. Role of fibronectin in the migration of fibroblasts into plasma clots. J Cell Biol. 1986. 102:2318–2323.

18. Rostagno AA, Schwarzbauer JE, Gold LI. Comparison of the fibrin-binding activities in the N- and C-termini of fibronectin. Biochem J. 1999. 338(Pt 2):375–386.

19. Matsuka YV, Medved LV, Brew SA, Ingham KC. The NH2-terminal fibrin-binding site of fibronectin is formed by interacting fourth and fifth finger domains. Studies with recombinant finger fragments expressed in Escherichia coli. J Biol Chem. 1994. 269:9539–9546.

20. Garcia-Pardo A, Pearlstein E, Frangione B. Primary structure of human plasma fibronectin. Characterization of a 31,000-dalton fragment from the COOH-terminal region containing a free sulfhydryl group and a fibrin-binding site. J Biol Chem. 1985. 260:10320–10325.

21. Cutler SM, Garcia AJ. Engineering cell adhesive surfaces that direct integrin alpha5beta1 binding using a recombinant fragment of fibronectin. Biomaterials. 2003. 24:1759–1770.

22. Kim TI, Jang JH, Chung CP, Ku Y. Fibronectin fragment promotes osteoblast-associated gene expression and biological activity of human osteoblast-like cell. Biotechnol Lett. 2003. 25:2007–2011.

23. Kim TI, Jang JH, Lee YM, Rhyu IC, Chung CP, Han SB, et al. Biomimetic approach on human periodontal ligament cells using synthetic oligopeptides. J Periodontol. 2004. 75:925–932.

24. Ku Y, Chung CP, Jang JH. The effect of the surface modification of titanium using a recombinant fragment of fibronectin and vitronectin on cell behavior. Biomaterials. 2005. 26:5153–5157.

25. Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997. 64:295–312.

26. Koch TG, Heerkens T, Thomsen PD, Betts DH. Isolation of mesenchymal stem cells from equine umbilical cord blood. BMC Biotechnol. 2007. 7:26.

27. Clover J, Gowen M. Are MG-63 and HOS TE85 human osteosarcoma cell lines representative models of the osteoblastic phenotype? Bone. 1994. 15:585–591.

28. Clark RA, An JQ, Greiling D, Khan A, Schwarzbauer JE. Fibroblast migration on fibronectin requires three distinct functional domains. J Invest Dermatol. 2003. 121:695–705.

29. Purohit A, Flanagan AM, Reed MJ. Estrogen synthesis by osteoblast cell lines. Endocrinology. 1992. 131:2027–2029.

30. Kinpara K, Mogi M, Kuzushima M, Togari A. Osteoclast differentiation factor in human osteosarcoma cell line. J Immunoassay. 2000. 21:327–340.

31. Rostagno A, Williams MJ, Baron M, Campbell ID, Gold LI. Further characterization of the NH2-terminal fibrin-binding site on fibronectin. J Biol Chem. 1994. 269:31938–31945.

32. Williams MJ, Phan I, Harvey TS, Rostagno A, Gold LI, Campbell ID. Solution structure of a pair of fibronectin type 1 modules with fibrin binding activity. J Mol Biol. 1994. 235:1302–1311.

33. Blystone SD, Weston LK, Kaplan JE. Fibronectin dependent macrophage fibrin binding. Blood. 1991. 78:2900–2907.

34. Magnusson MK, Mosher DF. Fibronectin: structure, assembly, and cardiovascular implications. Arterioscler Thromb Vasc Biol. 1998. 18:1363–1370.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download