Abstract
Purpose
The aim of this study was to define the immunoreactive specificity of Porphyromonas gingivalis (P. gingivalis) heat shock protein (HSP) 60 in periodontitis and atherosclerosis.
Methods
In an attempt to define the cross-reactive bacterial heat-shock protein with human self-antigen at molecular level, we have introduced a novel strategy for cloning hybridoma producing anti-P. gingivalis HSP 60 which is polyreactive to bacterial HSPs or to the human homolog.
Results
Five cross-reactive clones were obtained which recognized the #19 peptide (TLVVNRLRGSLKICAVKAPG) among 37 synthetic peptides (20-mer, 5 amino acids overlapping) spanning the whole molecule of P. gingivalis HSP 60. We have also established three anti-P. gingivalis HSP 60 monoclonal antibodies demonstrating mono-specificity. These clones recognized the #29 peptide (TVPGGGTTYIRAIAALEGLK).
The crucial role of Porphyromonas gingivalis (P. gingivalis) heat shock protein (HSP)60 in the immunopathogenic mechanism of both periodontitis and atherosclerosis has been demonstrated in the context of immunodominant T- and/or B-cell epitopes in our previous studies [1-4]. Also we have recently reported that vaccines formulated with P. gingivalis HSP60 can successfully reduce the alveolar bone loss in experimental periodontitis as a polymicrobial infection [5] and that anti-P. gingivalis HSP60 antibody manifested cross-species recognition to exert an opsonophagocytic function against multiple periodontopathogenic bacteria [6]. However, a concern about potential autoimmune reaction may arise inherent from the high level of sequence homology of bacterial HSP with human self-antigen when the whole HSP60 molecule is considered for vaccine administration.
Nonetheless, identifying a defined cross-reactive bacterial peptide antigen is of critical value in several ways including 1) for identifying the putative peptide antigen responsible for inducing or suppressing autoimmunity in host, 2) for identifying a candidate peptide vaccine to provide cross-species immunity, or 3) for use as a tool for mobilizing antigen-specific regulatory T cells to suppress autoimmune diseases. Thus, defining a peptide molecule from bacterial HSP60 that is cross-reactive with human HSP60 at the molecular level would be an exciting idea for stimulating antigen-specific regulatory T cells that might suppress a P. gingivalis HSP-triggered autoimmune response either in periodontitis as an infectious disease [7] or atherosclerosis as an autoimmune disease [8]. In the same way, identifying bacterial HSP60 peptide that exhibits cross-species recognition would facilitate peptide vaccine development for protection against periodontitis as a polymicrobial disease.
However, simply comparing sequence similarity (homology) or mapping immunodominant epitopes might provide only limited information on which peptide of P. gingivalis HSP does cross-react with human HSP peptide within the gingival lesion or arterial wall at the molecular level. To circumvent these underlying obstacles, we have adopted an innovative strategy to incorporate the monoclonal hybridoma technology to screen candidate peptides that may manifest poly-specificities to exogenous bacterial or to indigenous human self-antigens at molecular level. This concept stems from the polyreactive nature of antibodies to pathogen-associated molecular pattern such as HSP, lipopolysaccharide, or phosphorylcholine [9-11]. Identifying a P. gingivalis HSP peptide sequence recognized by the monoclonal antibody would thus enable us to clarify the exact immunodominant peptide epitope(s) responsible for eliciting in vivo cross-reactivity to its human counterpart.
The primary goal of the present study was to propose an innovative monoclonal hybridoma technology to define an immunodominant epitope of P. gingivalis HSP60 either mono-specific to its cognate antigen or poly-specific to other bacterial HSP's or with a human homolog,
Recombinant P. gingivalis HSP60 was purified from the P. gingivalis GroEL gene (a gift from Dr. Yoji Murayama, Okayama, Japan) as previously reported [2,6]. C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME, USA) were initially immunized subcutaneously with 50 g of recombinant P. gingivalis HSP60 emulsified in complete Freund's adjuvant followed by two subsequent subcutaneous injections of the HSP60 in incomplete Freund's adjuvant. Animals were bred and maintained in a specific-pathogen-free animal breeding facility, the experiments were conducted according to Declaration of Helsinki principles, and the experimental protocol was approved by the Institutional Review Board of Pusan National University Hospital.
Two weeks after the final immunization, mouse spleen cells were homogenized to a single cell by passing them through 30 µm nylon mesh (Miltenyi Biotech GmbH, Bergisch Gladbach, Germany) and suspended in serum-free Dulbecco's modification of eagle's medium (DMEM). The harvested cells were lysed of contaminating red blood cells and resuspended to fuse with the same number of mouse myeloma cells (SP2/0-Ag14, American Type Culture Collection #CRL 1581) in the presence of 50% polyethylene glycol. The fused cells were incubated in DMEM containing 20% fetal bovine serum, and then incubated in hypoxanthine-aminopterin-thymidine-containing media for 2 weeks to remove unfused cells. The selection procedure was terminated by adding hypoxanthine-thymidine-containing media. Wells with single foci of homogeneous cells were identified to collect culture supernatants and were screened for secretion of the anti-P. gingivalis HSP60 IgG antibody. To ensure the monoclonality of the cells, limiting dilution was performed down to 0.3 cell/well in 96-well plates until hybridoma producing anti-P. gingivalis HSP60 IgG antibody was finally identified.
Microtiter plates coated with P. gingivalis HSP60, diluted in 10 mM phosphate buffer [12-14], were incubated with an aliquot of cell culture supernatants. After samples were washed, horseradish peroxidase-conjugated goat anti-mouse IgG (γ-chain specific, Jackson ImmunoResearch Laboratories Inc., West Grove, PA, USA) was added. After incubation for 2 hours at room temperature, the plates were washed, and an aliquot of tetramethylbenzidine (Kirkegaard and Perry Laboratories, Gaithersburg, MD, USA) was added for incubation, followed by the addition of 0.18 M H2SO4 to stop the color development. Optical densities (OD) were plotted as a function of the serum dilution factor. Serum dilution factor corresponding to an optical density of 0.5 was assigned an ELISA unit. The wells producing antibody units higher than a mean OD+3×s.d. were identified as being positive.
The established hybridoma cells were expanded and 2×106 cells were injected into the peritoneal cavity of the C57BL/6 mouse that had been immune-suppressed previously by an intraperitoneal injection of immunosuppressant (500 µL/mouse, Pristane, Sigma-Aldrich Co., St. Louis, MO, USA). Ascites fluid was collected by needle aspiration and anti-P. gingivalis monoclonal IgG antibody was purified by using an immunoglobulin G purification system (ImmunoPure, Pierce Chemical Co., Rockford, IL, USA).
Five µg of recombinant P. gingivalis HSP60, 100 µg of lysate preparations from five bacterial species, and 5 µg of human HSP60 (StressGen Biotechnologies Co., Victoria, Canada) were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and electro-transferred to nitrocellulose membrane. After blocking the membrane, purified anti-P. gingivalis HSP60 monoclonal antibody was applied for incubation. The membrane was washed and orseradish peroxidase-conjugated mouse anti-mouse IgG was added. After washing the membrane, tetramethylbenzidine was added for visualization of bound antibodies. Bacterial cell lysates was prepared by sonicating five periodontopathic bacterial cells consisting of P. gingivalis, Actinobacillus actinomycetemcomitans, Prevotella intermedia, Treponema denticola, Streptococcus mutans, and Fusobacterium nucleatum that had been anaerobically grown and then heat-shocked for 1 hour at 44℃. We loaded 100 µg of bacterial cell lysate proteins, being equivalent to approximately 5 µg of HSP's, for semiquantitative comparison of blotting intensity. This method was derived from the idea that heat-inducible protein, representing 1 to 2% of total bacterial proteins under normal conditions, increases four- to five-fold under heat shock treatment [15].
A total of 37 overlapping peptides, each consisting of 20 amino acid with 5 amino acids overlapping, spanning whole molecule of P. gingivalis HSP60 were synthesized by Fmoc solid phase peptide synthesis using ASP48S (Peptron Inc., Daejeon, Korea) and purified by the reverse phase HPLC using a Vydac Everest C18 column. Elution was carried out with a water-acetonitrile linear gradient (10 to 75% (v/v) of acetonitrile) containing 0.1% (v/v) trifluoroacetic acid.
Briefly, each peptide (1 µg) was spotted onto polyvinyliden fluoride membrane. The membrane was blocked with 5% skim milk followed by adding the monoclonal antibody in phosphate buffered saline (PBS) buffer for incubation for 2 hours at room temperature. After washing the membrane, horseradish peroxidase-conjugated goat anti-mouse IgG was added for 1 hour. The membrane was then washed with PBS-Tween followed by adding tetramethylbenzidine for color development.
A total of eight anti-P. gingivalis HSP60 IgG monoclonal antibodies were cloned and each was subjected to SDS-PAGE for verification of purity consisting of heavy and light chain molecules of IgG antibody, respectively (Fig. 1).
Each purified monoclonal antibody was examined for its reactivity with recombinant P. gingivalis HSP60, bacterial HSP proteins, or human HSP60, respectively, by western immunoblot. Based on their mono- or poly-reactivity with different HSP's, two different panels of monoclonal antibodies have been categorized: one being mono-specific to cognate P. gingivalis HSP60, the other being poly-specific to different bacterial HSP's and human HSP60 (Figs. 2 and 3).
We were able to obtain eight clones from mouse hybridoma producing a panel of monoclonal IgG antibodies to P. gingivalis HSP60 which was used for the immunogenic antigen. Five clones (JC5-JC9) of poly-specific anti-P. gingivalis HSP60 monoclonal antibodies recognized #19 synthetic peptide, while three clones (JC1-JC3) of mono-specific anti-P. gingivalis HSP60 monoclonal antibodies recognized peptide #29 of synthetic peptide spanning the whole molecule of P. gingivalis HSP60, as evidenced by dot immunoblot analyses (Fig. 4). For further characterization of the five poly-reactive monoclonals, we have synthesized #19 peptides based on known sequences of human HSP60, Mycobacterium tuberculosis (M. tuberculosis), and Chlamydia pneumoniae (C. pneumoniae) and tested them for inter-species cross-reactivity with non-oral pathogens. In addition to reactivity with peptide #19 from human HSP60, all of the clones have demonstrated inter-species cross-reactivity with peptide #19 from C. pneumoniae, but not with that of M. tuberculosis as shown by dot immunoblot analysis (Fig. 5). The peptide specificities of cloned monoclonal antibodies are summarized in Table 1.
Despite the monoclonality of hybridoma cell lines established in the course of limiting dilution, five of them exhibited an identical pattern of polyreactivity with cognate HSP antigen and other bacterial HSP's, as well as to human homolog, while three of them demonstrated a monoreactivity to cognate HSP60 molecule. Thus, in this study it was possible to validate polyreactive monoclonal antibody, one of the characteristic features of host defense mechanisms against pathogen-associated molecular pattern, such as bacterial heat shock protein [9-11]. In an attempt to identify the specific epitope recognized by these monoclonal antibodies, we have synthesized 37 synthetic peptides (20 mer, 5 amino acids overlapping) spanning the whole molecule of P. gingivalis HSP60. Monoreactive monoclonal antibody to P. gingivalis HSP60 recognized peptide #29 (TVPGGGTTYIRAIAALEGLK), while polyreactive monoclonal antibody recognized peptide #19 (TLVVNRLRGSLKICAVKAPG). To confirm the cross-reactivity, we have synthesized peptide #19 from known sequences of human HSP60 molecule as well as lysate preparation from heat-induced whole bacterial cellular protein, which were used for immunoblot analysis. The cross-reactivity of the five monoclonal antibodies with synthetic peptide #19 from human HSP60 was confirmed by dot immunoblot. Here, we propose the monoclonal hybridoma technology as a novel strategy for elucidating cross-reactive immunodominant epitope(s) at a molecular level in autoimmune diseases.
Interestingly, based on our series studies and other report, peptide #19 has been immunodominant T-and B-cell epitope in both periodontitis and atherosclerosis patients [2,4,16,17] and common B-cell epitope in atherosclerosis patients [2], while peptide #29 has been B-cell epitope in periodontitis [12] and T- and B-cell epitope in atherosclerosis patients. Though our previous epitope mapping results may indicate that both peptide #19 and #29 might be involved in the immunopathologic process in atherosclerosis as an autoimmune disease, the latter may not be involved in the autoimmune pathologic process of atherosclerosis at the molecular level based on the monoclonal hybridoma technology-based approach introduced in our present study. The present study strongly suggests that peptide #19 may be an immunodominant epitope that demonstrates a robust cross-reactivity with other bacterial and human HSP60. Hence, peptide #19 may be responsible for either inducing or suppressing autoimmunity by the host, because cross-reactive peptide to self-antigen is known to mobilize antigen-specific regulatory T cells to suppress autoimmune diseases [18]. Potentially it could also be a candidate peptide for developing a vaccine to provide cross-species immunity in periodontitis as a polymicrobial infectious disease. HSP60 is widely known to be a molecular target for the T-cell immune response in the periodontitis-atherosclerosis link [2,8,19,20]. Hence, the polyclonal anti-P. gingivalis HSP60 antibody could exert either a protective or deteriorating effect on experimental periodontitis induced by multiple bacterial infection. Currently we have launched a series of studies aimed at evaluating the significant role of peptide #19 in the immunopathogenic mechanism of periodontitis or atherosclerosis.
Figures and Tables
Figure 1
Sodium dodecyl sulfate polyacrylamide gel electrophoresis profile of purified recombinant Porphyromonas gingivalis HSP60 (A) and one (clone JC2) of purified anti-P. gingivalis HSP60 monoclonal IgG antibody demonstrating the heavy and the light chain, respectively (B). MW: molecular weight marker, HSP: heat shock protein.
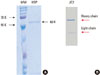
Figure 2
Reactive pattern of cross-reactive (A: clones JC5-JC9) and mono-reactive (B: clones JC1-JC3) or anti-Porphyromonas gingivalis heat shock protein 60 monoclonal antibodies with cognate antigen or with human counterpart demonstrated by immunoblot analysis.

Figure 3
Reactive pattern of poly-reactive (A: clones JC 5-JC9) and mono-reactive (B: clones JC1-JC3) anti-Porphyromonas gingivalis heat shock protein (HSP)60 monoclonal antibodies with cognate HSP antigen or with HSP's of five periodontopathogenic bacteria as demonstrated by immunoblot analysis. Pg: P. gingivalis HSP60, Aa: Actinobacillus actinomycetemcomitans, Pi: Prevotella intermedia, Fn: Fusobacterium nucleatum, Sm: Streptococcus mutans, Td: Treponema denticola.

Figure 4
Poly-reactive monoclonal antibody (A: clones JC5-JC9) recognized #19 peptide among 37 synthetic peptides spanning a whole molecule of Porphyromonas gingivalis heat shock protein (HSP)60 as well as human HSP60, while mono-reactive monoclonal antibody (B: clones JC1-JC3) recognized #29 peptide among the same synthetic peptides without responding to human HSP60, as evidenced by dot immunoblot analyses. Hu: human HSP60, Pg: P. gingivalis HSP60.

Figure 5
In addition to reactivity with peptide #19 (Hu#19) from self-antigen (human heat shock protein 60), inter-species cross-reactivity of the five monoclonal antibodies (clones JC5-JC9) with #19 synthetic peptides from Porphyromonas gingivalis (Pg#19), Mycobacterium tuberculosis (Myco#19) and Chlamydia pneumoniae (Chla#19), respectively, is demonstrated by dot immunoblot analysis. Note that none of them recognized #19 peptide from M. tuberculosis.

ACKNOWLEDGMENTS
The present study was supported by the Korea Healthcare Technology R&D Project, Ministry of Health & Welfare, Republic of Korea A080391, KOSEF RO1-2008-000-20044-0, and KRF E00130. The authors express our deep appreciation to Professors Eunyup Lee, Chulhoon Chang, and Hyunghoi Kim for their helpful advice and support in collecting blood samples.
References
1. Choi JI, Chung SW, Kang HS, Rhim BY, Kim SJ, Kim SJ. Establishment of Porphyromonas gingivalis heat-shock-protein-specific T-cell lines from atherosclerosis patients. J Dent Res. 2002. 81:344–348.

2. Choi JI, Chung SW, Kang HS, Rhim BY, Park YM, Kim US, et al. Epitope mapping of Porphyromonas gingivalis heat-shock protein and human heat-shock protein in human atherosclerosis. J Dent Res. 2004. 83:936–940.

3. Choi J, Chung SW, Kim SJ, Kim SJ. Establishment of Porphyromonas gingivalis-specific T-cell lines from atherosclerosis patients. Oral Microbiol Immunol. 2001. 16:316–318.

4. Choi JI, Kang HS, Park YM, Kim SJ, Kim US. Identification of T-cell epitopes of Porphyromonas gingivalis heat-shock-protein 60 in periodontitis. Oral Microbiol Immunol. 2004. 19:1–5.

5. Lee JY, Yi NN, Kim US, Choi JS, Kim SJ, Choi JI. Porphyromonas gingivalis heat shock protein vaccine reduces the alveolar bone loss induced by multiple periodontopathogenic bacteria. J Periodontal Res. 2006. 41:10–14.

6. Choi JI, Choi KS, Yi NN, Kim US, Choi JS, Kim SJ. Recognition and phagocytosis of multiple periodontopathogenic bacteria by anti-Porphyromonas gingivalis heat-shock protein 60 antisera. Oral Microbiol Immunol. 2005. 20:51–55.

7. Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nat Immunol. 2005. 6:353–360.

8. Van Eden W, Wick G, Albani S, Cohen I. Stress, heat shock proteins, and autoimmunity: how immune responses to heat shock proteins are to be used for the control of chronic inflammatory diseases. Ann N Y Acad Sci. 2007. 1113:217–237.

9. Lee J, Suh J, Choi J. B-1 cell-derived monoclonal antibodies and costimulatory molecules. J Surg Res. 2009. 154:293–298.

10. Notkins AL. Polyreactive antibodies and polyreactive antigen-binding B (PAB) Cells. Curr Top Microbiol Immunol. 2000. 252:241–249.

11. Zhou ZH, Tzioufas AG, Notkins AL. Properties and function of polyreactive antibodies and polyreactive antigen-binding B cells. J Autoimmun. 2007. 29:219–228.

12. Choi JI, Borrello MA, Cutler CW, Zauderer M. Prior Immunization with fusobacterium nucleatum interferes with opsonophagocytosis function of sera against Porphyromonas gingivalis. J Korean Acad Periodontol. 2000. 30:105–110.

13. Choi JI, Borrello MA, Smith ES, Zauderer M. Polarization of Porphyromonas gingivalis-specific helper T-cell subsets by prior immunization with Fusobacterium nucleatum. Oral Microbiol Immunol. 2000. 15:181–187.

14. Choi J, Borrello MA, Smith E, Cutler CW, Sojar H, Zauderer M. Prior exposure of mice to Fusobacterium nucleatum modulates host response to Porphyromonas gingivalis. Oral Microbiol Immunol. 2001. 16:338–344.

15. Shinnick TM. Heat shock proteins as antigens of bacterial and parasitic pathogens. Curr Top Microbiol Immunol. 1991. 167:145–160.

16. Maeda H, Miyamoto M, Kokeguchi S, Kono T, Nishimura F, Takashiba S, et al. Epitope mapping of heat shock protein 60 (GroEL) from Porphyromonas gingivalis. FEMS Immunol Med Microbiol. 2000. 28:219–224.

17. Bak J, Kim SJ, Choi J. Epitope specificity of Porphyromonas gingivalis heat shock protein for T-cell and/or B-cell in human atherosclerosis. J Korean Acad Periodontol. 2003. 33:179–191.

18. van Eden W, van der Zee R, Prakken B. Heat-shock proteins induce T-cell regulation of chronic inflammation. Nat Rev Immunol. 2005. 5:318–330.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download