Abstract
A number of surface modification techniques using immobilization of biofunctional molecules of Titanium (Ti) for dental implants as well as surface properties of Ti and Ti alloys have been developed. The method using passive surface oxide film on titanium takes advantage of the fact that the surface film on Ti consists mainly of amorphous or low-crystalline and non-stoichiometric TiO2. In another method, the reconstruction of passive films, calcium phosphate naturally forms on Ti and its alloys, which is characteristic of Ti. A third method uses the surface active hydroxyl group. The oxide surface immediately reacts with water molecules and hydroxyl groups are formed. The hydroxyl groups dissociate in aqueous solutions and show acidic and basic properties. Several additional methods are also possible, including surface modification techniques, immobilization of poly(ethylene glycol), and immobilization of biomolecules such as bone morphogenetic protein, peptide, collagen, hydrogel, and gelatin.
Metals have a long history in the treatment of dentistry. Titanium (Ti), in particular, is used for dental implants because of its high strength, toughness, and durability. However, titanium and other metals are typically artificial materials and have no biofunction, which makes them less attractive as biomaterials. To add biofunctionality to metals, surface modification is necessary because biofunctionality cannot be added during their manufacturing processes such as melting, casting, forging, and heat treatment. Surface modification is a process that changes a material's surface composition, structure, and morphology, leaving the bulk mechanical properties intact. In dentistry, dental implants require hard tissue compatibility for osseointegration and bone formation, soft tissue compatibility for adhesion of gingival epithelium, and antibacterial properties for the inhibition of biofilm formation. These biofunctional properties encompass a conflict between two properties: the enhancement and inhibition of protein adsorption or cell adhesion. For these purposes, especially for bone formation, many techniques for surface modification of metals have been attempted at the research stage and some of them have been commercialized. Reviews on the surface modification of Ti focusing on plasma spraying [1] and electrochemical treatments [2] have already been published. In this review, surface modification techniques using immobilization of biofunctional molecules of Ti for dental implants are categorized and explained. To develop surface modification techniques, it is first necessary to understand the surface properties of Ti and Ti alloys.
Except in reducing environments, corrosion processes cause a reaction film to form on metallic materials. Passive film is such a reaction film, which is of particular significance for corrosion protection. When the solubility is extremely low and pores are absent, the adhesion of film-which is formed in an aqueous solution-to the substrate will be strong. The film then becomes a corrosion-resistant film or a passive film. Due to the tremendously fast rate at which they are formed, passive films, which are 1 to 5 nm thick, readily become amorphous. For example, the film on a Ti metal substrate can be formed in 30 ms. Since amorphous films hardly contain grain boundaries or structural defects, they are usually corrosion resistant. However, corrosion resistance decreases with crystallization. Fortunately, passive films contain water molecules that promote and maintain amorphousness.
When Ti is polished in de-ionized water and analyzed using X-ray photoelectron spectroscopy, the Ti 2p spectrum obtained from the Ti gives four doublets, which correspond to the valences Ti0, Ti2+, Ti3+, and Ti4+, respectively, as shown in Fig. 1. A distinct Ti0 peak for the metallic state is observed, which accounts for a very thin surface oxide film, i.e., thinner than a few nanometers. Ti4+ (TiO2), Ti3+ (Ti2O3), and Ti2+ (TiO) are also detected. Though Ti2+ oxide exists in the surface oxide film, Ti2+ formation is always thermodynamically less favorable than Ti3+ formation at the surface. In the same specimen, the O 1s spectrum is observed, as shown in Fig. 2. The surface oxide contains a hydroxide or hydroxyl group (OH-) and water. The surface film on Ti consists mainly of amorphous or low-crystalline and non-stoichiometric TiO2, and the film resists chloride ions.
Reactions between the surfaces of metallic materials and living tissues are the initial events that occur when the matematerials are implanted into the human body. Tissue compatibility is governed by the reactions in the initial stage. Thus the surface properties of materials are important. The composition of the surface oxide film changes even though the film is macroscopically stable. Passive surfaces exist simultaneously in contact with electrolytes, undergoing a continuous process of partial dissolution and reprecipitation from the microscopic viewpoint [3]. Therefore, the surface composition should change according to the environment. Due to abrasion against bone and other materials, the surface oxide film might be scratched and destroyed during insertion and implantation into living tissues.
Calcium, phosphorus, and sulphur are incorporated into the surface film of Ti after it is surgically implanted into the human jaw [4]. Calcium phosphates are formed on Ti and its alloys by immersion in Hanks' solution and other solutions [5-9]. In fact, the above phenomena are characteristic of both Ti and its alloys [6].
Recently, zirconia has been used for dental implants, and its hard tissue compatibility has been claimed to be the same as that of Ti. This, however, is not the case. Zirconia is a bioinert ceramic; thus no chemical reaction in living tissues is expected. In addition, the surface oxide film on Ti is not completely oxidized, and is relatively reactive, while that on zirconium (Zr) is stably oxidized; that on Zr is more passive and protective than that on Ti. Neither calcium nor phosphate stably exists alone on Ti; stable, protective calcium phosphate is formed on Ti in biological environments [10]. On the other hand, calcium is never incorporated on Zr, while zirconium phosphate formed on Zr is highly stable and establishes a protective layer; therefore, no calcium reacts with the layer as shown in Fig. 3. Surface oxide films as passive films on Ti and Zr are nearly amorphous, and are different from crystallized titanium oxide and zirconium oxide bulk ceramics with regard to their chemical properties.
The surface of oxide reacts with moisture in air and hydroxyl groups are rapidly formed. In the case of Ti, the surface oxide immediately reacts not only with water molecules in aqueous solutions but also with moisture in air and is covered by hydroxyl groups [11,12]. The surface oxide is always formed on conventional metallic biomaterials and the surface of the surface oxide is active for the same reason described above. Therefore, the oxide surface immediately reacts with water molecules and hydroxyl groups are formed as shown in Fig. 4A. The surface hydroxyl groups contain both terminal OH and bridge OH in equal amounts. Concentration of hydroxyl groups on the unit area of the surface is determined with various techniques.
Active surface hydroxyl groups dissociate in aqueous solutions and form electric charges as shown in Fig. 4B [11-14]. Positive or negative charge due to the dissociation is governed by the pH of the surrounding aqueous solution: positive and negative charges are balanced and the apparent charge is zero at a certain pH. This pH is the point of zero charge (pzc). The pzc is the unique value for an oxide and an indicator that the oxide shows acidic or basic properties. For example, in the case of TiO2, the pzc of rutile is 5.3 and that of anatase is 6.2 [11]. In other words, the anatase surface is acidic at lower pH and basic at higher pH than 6.2. Active surface hydroxyl groups and electric charges formed by the dissociation of the groups play important roles in the bonding with polymers and immobilization of molecules. Therefore, the concentration of the surface hydroxyl group and the pH are important factors in the bonding with polymeric materials and immobilization of molecules.
In Table 1, surface modification techniques are categorized according to their processes and purposes. The major purpose of surface modification is to improve hard tissue compatibility or accelerate bone formation. Research to improve hard-tissue compatibility involves two approaches based on the resultant surface layer: a calcium phosphate and titanium oxide layer with the thickness measured in micrometers and a surface-modified layer with the thickness measured in nanometers. Most of these processes have been developed since the 1990s. Fig. 5 shows the history of the surface treatment technique to improve hard tissue compatibility.
Surface properties are particularly significant for biomaterials, and thus surface modification techniques are particularly useful for biomaterials. Dry processing (using ion beams) and wet processing (which is performed in aqueous solutions) are predominant surface modification techniques. In particular, among wet processes, the electrochemical technique has recently become important. Immobilization of bone formation factors such as bone morphological protein, bone morphogenetic protein (BMP), or biomolecules such as collagen and peptide to metal surfaces is another technique for improving hard tissue compatibility. On the other hand, the immobilization of biofunctional molecules such as poly(ethylene glycol), PEG, to the metal surface to control the adsorption of proteins and adhesion of cells, platelets, and bacteria has also been attempted.
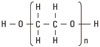
PEG is an oligomer or polymer of ethylene oxide, but historically, PEG has tended to refer to oligomers and polymers with a molecular weight below 20,000. PEG has the structure shown in Fig. 6. PEGylation is the act of covalently coupling a PEG structure to another larger molecule, for example, a therapeutic protein (which is then referred to as PEGylated). PEG is soluble in water, methanol, benzene, and dichloromethane and is insoluble in diethyl ether and hexane. It is coupled to hydrophobic molecules to produce non-ionic surfactants. This property, combined with the availability of PEGs with a wide range of end-functions, contributes to the wide use of PEGs in biomedical research: drug delivery, tissue engineering scaffolds, surface functionalization, and many other applications [15].
The immobilization of biofunctional polymers on noble metals such as Au is usually conducted by using the bonding -SH or -SS- group; however, this technique can only be used for noble metals. The adhesion of platelets and adsorption of proteins, peptides, antibodies, and DNA is controlled by modifications of the above technique. On the other hand, PEG is a biofunctional molecule on which adsorption of proteins is inhibited. Therefore, immobilization of PEG to the metal surface is an important event to bio-functionalize the metal surface. Examples of immobilization of PEG to an oxide surface are shown in Fig. 6. A class of copolymers based on poly(L-lysine)-g-poly(ethylene glycol), PLL-g-PEG, has been found to spontaneously adsorb from aqueous solutions onto TiO2, Si0.4Ti0.6O2, and Nb2O5 to develop blood-contacting materials and biosensors [16,17]. In another case, TiO2 and Au surfaces are functionalized by the attachment of poly(ethylene glycol)-poly(DL-lactic acid), copolymeric micelles. The micelle layer can enhance the resistance to protein adsorption to the surfaces up to 70% [18]. A surface of stainless steel was first modified by a silane-coupling agent (SCA), (3-mercaptopropyl) trimethoxysilane. The surface of the silanized stainless steel, SCA-SS, was subsequently activated by argon plasma and then subjected to UV-induced graft polymerization of poly(ethylene glycol)methacrylate (PEGMA). The PEGMA graft-polymerized stainless-steel coupon, PEGMA-g-SCA-SS, with a high graft concentration and, thus, a high PEG content was found to be very effective in preventing the absorption of bovine serum albumin and γ-globulin [19]. These processes require several steps, but are effective for immobilization; however, no promising technique for the immobilization of PEG to a metal surface has been developed so far. Photoreactive PEG can be photoimmobilized on Ti [20].
Both terminals of PEG (MW: 1000) are terminated with-NH2 (NH2-PEG-H2), but only one terminal is terminated with -NH2 (NH2-PEG). The cathodic potential was charged to Ti from the open circuit potential to -0.5 V vs. a saturated calomel electrode and is maintained at this potential for 300 seconds. During charging, the terminated PEGs electrically migrats to and are deposited on the Ti cathode, as shown in Fig. 7. Not only electrodeposition but also immersion leads to the immobilization of PEG onto a Ti surface. However, more terminated amines combine with Ti oxide as an NH-O bond by electrodeposition, while more amines randomly exist as NH3+ in the PEG molecule by immersion (Fig. 8) [21,22]. A scanning probe microscopic image is shown in Fig. 9. The amounts of the PEG layer immobilized onto the metals are governed by the concentrations of the active hydroxyl groups on each surface oxide in the case of electrodeposition, which is governed by the relative permittivity of the surface oxide in the case of immersion [23]. The PEG-immobilized surface inhibits the adsorption of proteins and cells, as well as the adhesion of platelets [24] and bacteria [25] (Fig. 10), indicating that this electrodeposition technique is useful for the biofunctionalization of metal surfaces. It is also useful for all electroconductive materials and materials having complex surface topography.
Organic coating technology, which is based on the latest medical and cellular biological results, employs biopolymers (proteins) that have been immobilized on the surface of metallic implants. The intention is to reduce the region character of the implant for the body. This is accomplished by coating with substances that are normally found on the surface or in the vicinity of the tissue that has to be substituted by the implant. It has been found that these coatings act as local mediators of cell adhesion and, in consequence, as a stimulating factor for the growth and proliferation of the cells normally found around the substituted tissue. The tight attachment at the oxide-coated surface of the metallic implant and the conservation of the biological function of the proteins involved are prerequisites for obtaining these highly desirable properties.
Since the natural environment around the implant is aqueous while the surface of the implant is either bare or oxidized metal, specific demands are imposed on the coating in order to mediate successfully between these different structural entities. The purpose of these demands is to obtain the native conformation of all proteins and cells that are in contact with the coating and to avoid all forms of aggregation and other conformational changes that might lead to protein denaturalization or cell death.
One approach is the immobilization of biological molecules (growth factors, adhesive proteins) onto the implant surface in order to induce a specific cellular response and promote osseointegration. The application of large extracellular matrix proteins, however, can be unpractical due to their low chemical stability, solubility in biological fluids and high cost. In addition, entire extracellular matrix molecules are usually of allogenetic or xenogenetic origin and thus associated with the risk of immune reaction and pathogen transfer. To immobilize biomolecules to metal surfaces, the following techniques are used: modification through silanized titania (thiol-directed immobilization; amino- and carboxyl-directed immobilization), modification through photochemistry, electrochemical techniques, and chemical modifications besed on self-assembled monolayers.
The immobilization of biomolecules to metallic surface can be achieved using self-assembled monolayers as cross-linkers. Self-assembled monolayers provide chemically and structurally well-defined surfaces that can often be manipulated using standard synthetic methodologies [26]. Thiol-anchored self-assembled monolayers [27,28] and siloxane-anchored self-assembled monolayers [29] have been particularly well-studied. A problem related to the application of immobilized biomolecules via silanization techniques is the hydrolysis of siloxane films when exposed to aqueous (physiological) conditions [30]. More recently, alkyl phosphate films that retain robust under physiological conditions [31] have been used to provide an ordered monolayer on tantalum oxide surfaces [32,33], and alkalphosphonic acids have been used to coat the native oxide surfaces of metals and their alloys inducing iron [34], steel [35], and Ti [36].
In a living tissue, the most important role played by the extracelluar matrix has been highlighted to favor cell adhesion [37]. Studies have shown that interactions occur between cell membrane receptors and adhesion proteins (or synthetic peptides) derived from the bone matrix, such as type I collagen or fibronectin [38]. These proteins are characterized by a Arg-Gly-Asp (RGD) motif which creates special transmembrane connections between the actin cytoskeleton and the RGD motif, and the whole system can activate several intracellular signaling pathways modulating cell behavior (e.g., proliferation, apoptosis, shape, mobility, gene expression, and differentiation) [39].
Due to the main role of the RGD sequence in cell adhesion, several research groups have developed biofunctionalized surfaces by immobilization of RGD peptides. Grafting RGD peptides has been performed on different biomaterials, such as Ti [40-42] and has been shown to improve osteoconduction in vitro. Methodologies differ by the conformation of RGD (cyclic or linear) and by the technique used for peptide immobilization [37,38,41-43]. Since the graft of a RGD peptide is known to be efficient in bone reconstruction [44], the challenge is to develop simple and cheap methods to favor cell anchorage on biomaterial surfaces [42,43].
Self assembled molecular monolayers bearing RGD moieties have been grafted to numerous surfaces, using either silanes [45], phosphonates on oxidized surfaces [42], or thiols on gold (Au) [43], but still have some application problems for large scale production. Phosphonates are known to adsorb on Ti. To be mechanically and physiologically stable, phosphonate layers have to be covalently bound to the material surface by using drastic conditions [36,46] which are not compatible with biomolecule stability. Monolayers of RGD-phosphonates have been achieved using a complex multistep process which necessitates to tether a primer onto the Ti surface, then a linker, and finally the peptide [47]. To immobilize RGD to the electrodeposited PEG on Ti, PEG with an -NH2 group and a -COOH group (NH2-PEG-COOH) must be employed. One terminal group, -NH2, is required to bind stably with a surface oxide on a metal. On the other hand, the other terminal group, -COOH, is useful to bond biofunctional molecules such as RGD as shown in Fig. 11 [48]. This RGD/PEG/Ti surface accelerates calcification by MC3T3-E1 cells [49]. The calcification is the most extensive on the RGD/PEG/Ti surface (Fig. 12). The bone healing of on the RGD/PEG/Ti surface implanted in rabbit tibia is better than that on RGD/Ti surface [50].
Glycine (G)-arginine (R)-glycine (G)-asparaginic acid (D)-serine (S) sequence peptide, GRGDS peptide, is coated with chloride activation technique to enhance adhesion and migration of osteoblastic cells [51]. The expression levels of many genes in MC3T3-E1 cells are altered.
Among the relevant molecules involved in biochemical modification of bone-contacting surfaces, growth factor, such as BMP-2, is of primary interest. BMP-2 has been known to play an important role in bone healing processes and to enhance therapeutic efficiency. Ectopic bone formation by BMP-2 in animals has been well established following the first reports of BMP-2 by the a research group [52-54]. Synthetic receptor binding motif mimicking BMP-2 is covalently linked to Ti surfaces through a chemical conjunction process [55]. A complete and homogeneous peptide overlayer on the Ti surfaces; the content is further measured by gamma counting. Biological evaluations show that the biochemically modified Ti were effective in terms of cell attachment behavior. Ti surfaces can enhance the rate of bone healing as compared with untreated Ti surface. Bone morphogenetic protein-4 (BMP-4) is immobilized on a Ti-6Al-4V alloy through lysozyme to improve the hard tissue response [56]. Proteins are silane-coupled to the oxidized surfaces of the Co-Cr-Mo alloy, the Ti-6Al-4V alloy, Ti, and the Ni-Ti alloy to improve tissue compatibility [57].
Type I collagen is immobilized by immersion in the collagen solution [58]. Type I collagen production increases with modification by ethane-1,1,2-triphosphonic acid and methylenediphosphonic acid grafted onto Ti [59]. Type I collagen is grafted through glutaraldehyde as a crosslinking agent [60]. For the electrodeposition, it is found that an alternating current between -1 V and + 1V vs. saturated calomel electrode with 1 Hz is effective to immobilize type I collagen to Ti and durability in water is high [61]. The immobilized collagen fiber network image is shown in Fig. 13.
Immobilization or coating of hydrogel to metal surface is currently attempting to add a drug delivery ability to orthopedic implant and stents or fluorescent sensing ability to microchips. Currently, synthetic polymeric hydrogels like poly (hydroxyethylmethacrylate) (pHEMA) and poly(hydroxyethylacrylate) are widely used as compliant materials particularly in the case of contact with blood or other biological fluids [64]. Despite hydrogel good flexibility in the swollen state, hydrogels usually lack of suitable mechanical properties and this could greatly impair their use as coating materials for surgical procedure. Moreover, in case of inadequate adhesion between the hydrogel coating and the metal surface, a breakage at the coating-steel interface might occur [65]. A spray coated method has been set up with the aim to control the coating of pHEMA onto the complex surface of a 316L steel stent for percutaneous coronary intervention [66]. The pHEMA coating evaluation of roughness wettability together with its morphological and chemical stability after three cycles of expansion-crimping along with preliminary results after 6 months demonstrates the suitability of the coating for surgical implantation of stent.
An alternative very promising synthetic route is represented by electrochemical polymerization, which leads to thin film coatings directly on the metal substrates with interesting applications either for corrosion protection or for the development of bioactive films [67-70]. As far as orthopedic field is concerned, in recent years, many procedures based on surface modification have been suggested to improve the biocompatibility and biofunction of Ti-based implant [71]. 2-Hydroxy-ethyl-methacrylate, a macromer poly(ethylene-glycol diacrylate) (PEGDE) and PEGDE copolymerized with acrylic acid were used to obtain hydrogels. A model protein and a model drug were entrapped in the hydrogel and released according to pH change [72].
Figures and Tables
Figure 1
Decomposition of titanium (Ti) 2p XPS spectrum obtained from titanium abraded and immersed for 300 seconds in water into eight peaks (2p3/2 and 2p1/2 electron peaks in four valences). Numbers with arrows are valence numbers.

Figure 2
Typical O 1s spectrum obtained from polished titanium and its de-convolution into O2-, OH-, and H2O components.

Figure 3
Neither calcium nor phosphate stably exists alone on titanium (Ti); stable, protective calcium phosphate is formed on Ti in biological environments. On the other hand, calcium is never incorporated on zirconium (Zr), while zirconium phosphate formed on Zr is highly stable and establishes a protective layer; therefore, no calcium reacts with the layer.

Figure 4
Formation process of hydroxyl group on titanium oxide (A) and dissociation of the hydroxyl group in aqueous solution and point of zero charge (pzc) (B).

Figure 5
History of surface treatment technique to improve hard tissue compatibility. Approaches to improving hard-tissue compatibility are categorized based on the resultant surface layer: calcium phosphate layer formation with thickness measured in micrometers and surface modified layer formation with thickness measured in nanometers. RF: radio frequency.
Figure 7
Attraction of poly(ethylene glycol) (PEG) with positively charged terminal to cathodic Ti surface by electrodeposition.
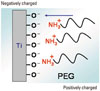
Figure 8
Schematic model of the deposition manner and chemical bonding state of poly(ethylene glycol) (PEG) by immersion and electrodeposition. (Modified from Tanaka Y, Doi H, Iwasaki Y, Hiromoto S, Yoneyama T, Asami K, et al. Mater Sci Eng C-Biom Supramol Syst 2007;27:206-12, with permission of Elsevier) [21].

Figure 9
Scanning probe microscopic image of electrodeposited poly(ethylene glycol) (PEG) to titanium surface.

Figure 10
Platelet adhesion and fibrin network formation (A1) and bacterial adhesion (A2) are active on titanium (Ti), while they are inhibited on poly(ethylene glycol)-electrodeposited Ti surfaces (B1 and 2).
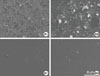
Figure 11
Poly(ethylene glycol) (PEG) twitter ion is electrodeposited to titanium (Ti) firstly and Arg-Gly-Asp (RGD) is immobilized on the PEG. (Modified from Tanaka Y, Saito H, Tsutsumi Y, Doi H, Nomura N, Imai H, et al. J Colloid Interface Sci 2009;330:138-43, with permission of Elsevier) [48].
Figure 12
Calcification (dark regions) by MC3T3-E1 cells are more active on Arg-Gly-Asp (RGD)/poly(ethylene glycol) (PEG)/titanium (Ti) specimen than on RGD/Ti and Ti. Scale bar represents 5 mm.

Figure 13
Scanning probe microscopic image of collagen electrodeposited on titanium with an alternating potential.

ACKNOWLEDGEMENTS
This review is written based on a Special Lecture in 2011 Spring Scientific Meeting of Korea Academy of Periodontology.
References
1. Yang Y, Kim KH, Ong JL. A review on calcium phosphate coatings produced using a sputtering process--an alternative to plasma spraying. Biomaterials. 2005. 26:327–337.

2. Kim KH, Ramaswamy N. Electrochemical surface modification of titanium in dentistry. Dent Mater J. 2009. 28:20–36.

3. Kelly EJ. Electrochemical-behavior of titanium. Mod Asp Electrochem. 1982. 14:319–424.
4. Sundgren JE, Bodo P, Lundstrom I. Auger-electron spectroscopic studies of the interface between human-tissue and implants of titanium and stainless-steel. J Colloid Interface Sci. 1986. 110:9–20.

5. Hanawa T, Ota M. Calcium phosphate naturally formed on titanium in electrolyte solution. Biomaterials. 1991. 12:767–774.

6. Hanawa T. Davies JE, editor. Titanium and Its Oxide Film: a substrate for formation of apatite. The bone-biomaterial interface. 1991. Toronto: University of Toronto Press;49–61.
7. Hanawa T, Ota M. Characterization of surface-film formed on titanium in electrolyte using XPS. Appl Surf Sci. 1992. 55:269–276.

8. Hanawa T, Okuno O, Hamanaka H. Compositional change in surface of TI-ZR alloys in artificial bioliquid. J Jpn Inst Met. 1992. 56:1168–1173.

9. Hiromoto S, Hanawa T, Asami K. Composition of surface oxide film of titanium with culturing murine fibroblasts L929. Biomaterials. 2004. 25:979–986.

10. Tsutsumi Y, Nishimura D, Doi H, Nomura N, Hanawa T. Difference in surface reactions between titanium and zirconium in Hanks' solution to elucidate mechanism of calcium phosphate formation on titanium using XPS and cathodic polarization. Mater Sci Eng C-Biom Supramol Syst. 2009. 29:1702–1708.

12. Westall J, Hohl H. A comparison of electrostatic models for the oxide/solution interface. Adv Colloid Interface Sci. 1980. 12:265–294.

13. Healy TW, Fuerstenau DW. The oxide-water interface-Interreaction of the zero point of charge and the heat of immersion. J Colloid Sci. 1965. 20:376–386.

14. Boehm HP. Acidic and basic properties of hydroxylated metal oxide surfaces. Discuss Faraday Soc. 1971. (52):264–275.

15. Mahato RI. Biomaterials for delivery and targeting of proteins and nucleic acids. 2005. Boca Raton: CRC Press.
16. Kenausis GL, Voros J, Elbert DL, Huang NP, Hofer R, Ruiz-Taylor L, et al. Poly(L-lysine)-g-poly(ethylene glycol) layers on metal oxide surfaces: attachment mechanism and effects of polymer architecture on resistance to protein adsorption. J Phys Chem. 2000. 104:3298–3309.

17. Huang NP, Michel R, Voros J, Textor M, Hofer R, Rossi A, et al. Poly(L-lysine)-g-poly(ethylene glycol) layers on metal oxide surfaces: surface-analytical characterization and resistance to serum and fibrinogen adsorption. Langmuir. 2001. 17:489–498.

18. Huang NP, Csucs G, Emoto K, Nagasaki Y, Kataoka K, Textor M, et al. Covalent attachment of novel poly(ethylene glycol)-poly(DL-lactic acid) copolymeric micelles to TiO2 surfaces. Langmuir. 2002. 18:252–258.

19. Zhang F, Kang ET, Neoh KG, Wang P, Tan KL. Surface modification of stainless steel by grafting of poly(ethylene glycol) for reduction in protein adsorption. Biomaterials. 2001. 22:1541–1548.

20. Ito Y, Hasuda H, Sakuragi M, Tsuzuki S. Surface modification of plastic, glass and titanium by photoimmobilization of polyethylene glycol for antibiofouling. Acta Biomater. 2007. 3:1024–1032.

21. Tanaka Y, Doi H, Iwasaki Y, Hiromoto S, Yoneyama T, Asami K, et al. Electrodeposition of amine-terminated poly (ethylene glycol) to titanium surface. Mater Sci Eng C-Biom Supramol Syst. 2007. 27:206–212.

22. Tanaka Y, Doi H, Kobayashi E, Yoneyama T, Hanawa T. Determination of the immobilization manner of amine-terminated poly(ethylene glycol) electrodeposited on a titanium surface with XPS and GD-OES. Mater Trans. 2007. 48:287–292.

23. Tanaka Y, Saito H, Tsutsumi Y, Doi H, Imai H, Hanawa T. Active hydroxyl groups on surface oxide film of titanium, 316L stainless steel, and cobalt-chromium-molybdenum alloy and its effect on the immobilization of poly(ethylene glycol). Mater Trans. 2008. 49:805–811.

24. Tanaka Y, Matsuo Y, Komiya T, Tsutsumi Y, Doi H, Yoneyama T, et al. Characterization of the spatial immobilization manner of poly(ethylene glycol) to a titanium surface with immersion and electrodeposition and its effects on platelet adhesion. J Biomed Mater Res A. 2010. 92:350–358.

25. Tanaka Y, Matin K, Gyo M, Okada A, Tsutsumi Y, Doi H, et al. Effects of electrodeposited poly(ethylene glycol) on biofilm adherence to titanium. J Biomed Mater Res A. 2010. 95:1105–1113.

26. Balachnder N, Sukenik CN. Monolayer transformation by nucleophilic-substitution- applications to the creation of new monolayer assemblies. Langmuir. 1990. 6:1621–1627.

27. Bain CD, Troughton EB, Tao YT, Evall J, Whitesides GM, Nuzzo RG. Formation of monolayer films by the spontaneous assembly of organic thiols from solution onto gold. J Am Chem Soc. 1989. 111:321–335.

28. Dubois LH, Nuzzo RG. Synthesis, structure, and properties of model organic-surfaces. Ann Rev Phys Chem. 1992. 43:437–463.
30. Xiao SJ, Textor M, Spencer ND, Sigrist H. Covalent attachment of cell-adhesive, (Arg-Gly-Asp)-containing peptides to titanium surfaces. Langmuir. 1998. 14:5507–5516.

31. Gawalt ES, Avaltroni MJ, Danahy MP, Silverman BM, Hanson EL, Midwood KS, et al. Bonding organics to Ti alloys: facilitating human osteoblast attachment and spreading on surgical implant materials. Langmuir. 2003. 19:200–204.

32. Brovelli D, Hahner G, Ruis L, Hofer R, Kraus G, Waldner A, et al. Highly oriented, self-assembled alkanephosphate monolayers on tantalum(V) oxide surfaces. Langmuir. 1999. 15:4324–4327.

33. Textor M, Ruiz L, Hofer R, Rossi K, Feldman K, Hahner G, et al. Structural chemistry of self-assembled monolayers of octadecylphosphoric acid on tantalum oxide surfaces. Langmuir. 2000. 16:3257–3271.

34. Fang JL, Wu NJ, Wang ZW, Li Y. XPS, AES and Raman studies of an antitarnish film on tin. Corrosion. 1991. 47:169–173.

35. Van Alsten JG. Self-assembled monolayers on engineering metals: structure, derivatization, and utility. Langmuir. 1999. 15:7605–7614.

36. Gawalt ES, Avaltroni MJ, Koch N, Schwartz J. Self-assembly and bonding of alkanephosphonic acids on the native oxide surface of titanium. Langmuir. 2001. 17:5736–5738.

37. Verrier S, Pallu S, Bareille R, Jonczyk A, Meyer J, Dard M, et al. Function of linear and cyclic RGD-containing peptides in osteoprogenitor cells adhesion process. Biomaterials. 2002. 23:585–596.

38. Reyes CD, Petrie TA, Burns KL, Schwartz Z, García AJ. Biomolecular surface coating to enhance orthopaedic tissue healing and integration. Biomaterials. 2007. 28:3228–3235.

39. Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002. 110:673–687.
40. Bagno A, Piovan A, Dettin M, Chiarion A, Brun P, Gambaretto R, et al. Human osteoblast-like cell adhesion on titanium substrates covalently functionalized with synthetic peptides. Bone. 2007. 40:693–699.

41. Elmengaard B, Bechtold JE, Søballe K. In vivo study of the effect of RGD treatment on bone ongrowth on press-fit titanium alloy implants. Biomaterials. 2005. 26:3521–3526.

42. Rammelt S, Illert T, Bierbaum S, Scharnweber D, Zwipp H, Schneiders W. Coating of titanium implants with collagen, RGD peptide and chondroitin sulfate. Biomaterials. 2006. 27:5561–5571.

43. Auernheimer J, Zukowski D, Dahmen C, Kantlehner M, Enderle A, Goodman SL, et al. Titanium implant materials with improved biocompatibility through coating with phosphonate-anchored cyclic RGD peptides. Chembiochem. 2005. 6:2034–2040.

44. Ferris DM, Moodie GD, Dimond PM, Gioranni CW, Ehrlich MG, Valentini RF. RGD-coated titanium implants stimulate increased bone formation in vivo. Biomaterials. 1999. 20:2323–2331.

45. Xiao SJ, Textor M, Spencer ND, Wieland M, Keller B, Sigrist H. Immobilization of the cell-adhesive peptide Arg-Gly-Asp-Cys (RGDC) on titanium surfaces by covalent chemical attachment. J Mater Sci Mater Med. 1997. 8:867–872.
46. Silverman BM, Wieghaus KA, Schwartz J. Comparative properties of siloxane vs phosphonate monolayers on a key titanium alloy. Langmuir. 2005. 21:225–228.

47. Schwartz J, Avaltroni MJ, Danahy MP, Silverman BM, Hanson EL, Schwarzbauer JE, et al. Cell attachment and spreading on metal implant materials. Mater Sci Eng C-Biom Supramol Syst. 2003. 23:395–400.

48. Tanaka Y, Saito H, Tsutsumi Y, Doi H, Nomura N, Imai H, et al. Effect of pH on the interaction between zwitterions and titanium oxide. J Colloid Interface Sci. 2009. 330:138–143.

49. Oya K, Tanaka Y, Saito H, Kurashima K, Nogi K, Tsutsumi H, et al. Calcification by MC3T3-E1 cells on RGD peptide immobilized on titanium through electrodeposited PEG. Biomaterials. 2009. 30:1281–1286.

50. Park JW, Kurashima K, Tustusmi Y, An CH, Suh JY, Doi H, et al. Bone healing of commercial oral implants with RGD immobilization through electrodeposited poly(ethylene glycol) in rabbit cancellous bone. Acta Biomater. 2011. 7:3222–3229.

51. Yamamichi N, Pugdee K, Chang WJ, Lee SY, Yoshinari M, Hayakawa T, et al. Gene expression monitoring in osteoblasts on titanium coated with fibronectin-derived peptide. Dent Mater J. 2008. 27:744–750.

53. Lee YM, Nam SH, Seol YJ, Kim TI, Lee SJ, Ku Y, et al. Enhanced bone augmentation by controlled release of recombinant human bone morphogenetic protein-2 from bioabsorbable membranes. J Periodontol. 2003. 74:865–872.

54. Wikesjö UM, Lim WH, Thomson RC, Cook AD, Wozney JM, Hardwick WR. Periodontal repair in dogs: evaluation of a bioabsorbable space-providing macroporous membrane with recombinant human bone morphogenetic protein-2. J Periodontol. 2003. 74:635–647.

55. Seol YJ, Park YJ, Lee SC, Kim KH, Lee JY, Kim TI, et al. Enhanced osteogenic promotion around dental implants with synthetic binding motif mimicking bone morphogenetic protein (BMP)-2. J Biomed Mater Res A. 2006. 77:599–607.

56. Puleo DA, Kissling RA, Sheu MS. A technique to immobilize bioactive proteins, including bone morphogenetic protein-4 (BMP-4), on titanium alloy. Biomaterials. 2002. 23:2079–2087.

57. Nanci A, Wuest JD, Peru L, Brunet P, Sharma V, Zalzal S, et al. Chemical modification of titanium surfaces for covalent attachment of biological molecules. J Biomed Mater Res. 1998. 40:324–335.

58. Nagai M, Hayakawa T, Fukatsu A, Yamamoto M, Fukumoto M, Nagahama F, et al. In vitro study of collagen coating of titanium implants for initial cell attachment. Dent Mater J. 2002. 21:250–260.

59. Viornery C, Guenther HL, Aronsson BO, Péchy P, Descouts P, Grätzel M. Osteoblast culture on polished titanium disks modified with phosphonic acids. J Biomed Mater Res. 2002. 62:149–155.

60. Chang WJ, Ou KL, Lee SY, Chen JY, Abiko Y, Lin CT, et al. Type I collagen grafting on titanium surfaces using low-temperature glow discharge. Dent Mater J. 2008. 27:340–346.

61. Kamata H, Suzuki S, Tanaka Y, Tsutsumi Y, Doi H, Nomura N, et al. Effects of pH, potential, and deposition time on the durability of collagen electrodeposited to titanium. Mater Trans. 2011. 52:81–89.

62. Pugdee K, Shibata Y, Yamamichi N, Tsutsumi H, Yoshinari M, Abiko Y, et al. Gene expression of MC3T3-E1 cells on fibronectin-immobilized titanium using tresyl chloride activation technique. Dent Mater J. 2007. 26:647–655.

63. Abe Y, Hiasa K, Takeuchi M, Yoshida Y, Suzuki K, Akagawa Y. New surface modification of titanium implant with phospho-amino acid. Dent Mater J. 2005. 24:536–540.

64. Cadotte AJ, DeMarse TB. Poly-HEMA as a drug delivery device for in vitro neural networks on micro-electrode arrays. J Neural Eng. 2005. 2:114–122.

65. Belkas JS, Munro CA, Shoichet MS, Johnston M, Midha R. Long-term in vivo biomechanical properties and biocompatibility of poly(2-hydroxyethyl methacrylate-co-methyl methacrylate) nerve conduits. Biomaterials. 2005. 26:1741–1749.

66. Indolfi L, Causa F, Netti PA. Coating process and early stage adhesion evaluation of poly(2-hydroxy-ethyl-methacrylate) hydrogel coating of 316L steel surface for stent applications. J Mater Sci Mater Med. 2009. 20:1541–1551.

67. Fenelon AM, Breslin CB. The electropolymerization of pryrole at a CuNi electrode: corrosion protection properties. Corros Sci. 2003. 45:2837–2850.

68. Mengoli G. Feasibility of polymer film coatings through electroinitiated polymerization in aqueous medium. Adv Polym Sci. 1979. 33:1–31.

69. De Giglio E, Guascito MR, Sabbatin L, Zambonin G. Electropolymerization of pyrrole on titanium substrates for the future development of new biocompatible surfaces. Biomaterials. 2001. 22:2609–2616.

70. Rammelt U, Nguyen PT, Plieth W. Corrosion protection by ultrathin films of conducting polymers. Electrochimica Acta. 2003. 48:1257–1262.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download