Abstract
Purpose
Immediate implantation presents challenges regarding site healing, osseointegration, and obtaining complete soft-tissue coverage of the extraction socket, especially in the posterior area. This last issue is addressed herein using the double-membrane (collagen membrane+high-density polytetrafluoroethylene [dPTFE] membrane) technique in two clinical cases of posterior immediate implant placement.
Methods
An implant was placed immediately after atraumatically extracting the maxillary posterior tooth. The gap between the coronal portion of the fixture and the adjacent bony walls was filled with allograft material. In addition, a collagen membrane (lower) and dPTFE membrane (upper) were placed in a layer-by-layer manner to enable the closure of the extraction socket without a primary flap closure, thus facilitating the preservation of keratinized mucosa. The upper dPTFE membrane was left exposed for 4 weeks, after which the membrane was gently removed using forceps without flap elevation.
Results
There was considerable plaque deposition on the outer surface of the dPTFE membrane but not on the inner surface. Moreover, scanning electron microscopy of the removed membrane revealed only a small amount of bacteria on the inner surface of the membrane. The peri-implant tissue was favorable both clinically and radiographically after a conventional dental-implant healing period.
There are several options for the timing of implant placement following extraction of a tooth. The immediate placement of an implant into the extraction socket has been proposed to improve patient comfort and shorten the treatment period. This is most commonly indicated when tooth extraction is due to trauma, endodontic lesion, root fracture, or extensive decay, and when the bony walls of the alveolus remain intact.
When implants are placed at the time of tooth extraction, there is often a gap between the walls of the extraction socket and the implant. In addition, there is a soft opening and a lack of mucosa in the extraction socket itself. These hard-tissue gaps and the soft-tissue opening are usually wide in the posterior area, especially on the maxilla. Therefore, immediate implantation presents challenges for site healing and osseointegration. Obtaining complete soft tissue coverage of the extraction socket is also problematic, especially in the posterior area. Various techniques and materials have been developed and used to enhance bone formation and osseointegration within these sockets at the time of implant placement. The gap problem is relatively easily solved by filling the gap with graft materials and applying a membrane. Although small peri-implant bone defects can be completely healed without using guided bone regeneration (GBR) procedures [1], gaps exceeding 2 mm need to be grafted [2,3].
As stated above, one of the problems encountered is insufficient soft tissue to completely cover the GBR site, which usually makes it necessary to perform primary closure of the socket in order to protect the healing site from the oral environment. The use of bioabsorbable and nonabsorbable membranes usually necessitates primary closure over the socket, a requirement that increases surgical complexity. Moreover, although it is considered advisable to use a pedicled flap or a connective-tissue graft to achieve primary closure, this technique is not easy and is uncomfortable for the patient.
Therefore, a membrane made of high-density polytetrafluoroethylene (dPTFE)-which does not require primary closure has been introduced to obviate the need for such primary closure [4]. In addition, successful outcomes have been demonstrated when using this membrane in both animal and clinical investigations [5-7]. A recent study investigated the clinical regeneration of extraction sockets treated using dPTFE membranes without a graft material. This technique consistently led to the preservation of both the soft and hard tissues in the extraction sites [8]. Histologic evaluation indicated that the newly formed tissue in the extraction site was mainly regular trabecular bone with areas of bone marrow and typical cells. This tissue is similar to bone found in healed extraction sites [9]. One advantage of using dPTFE membranes is that no primary coverage is necessary, removing the need for additional releasing incisions and hence improving the surgical procedure by leaving the mucogingival junction unchanged. Moreover, the smooth surface of dPTFE membranes makes their removal easy, without the need for additional surgical interventions.
However, a limitation of the dPTFE membrane is its lack of tissue adhesion, which weakens its function as a membrane, and thus jeopardizes bone regeneration below the dPTFE membrane. The addition of a collagen membrane below the dPTFE membrane may enhance bone regeneration. This procedure is demonstrated herein with two clinical cases of posterior immediate implant placement achieved using the double-membrane (collagen membrane+dPTFE membrane) technique to perform GBR around installed implants without primary flap closure, thus facilitating the preservation of the keratinized mucosa.
After local anesthesia, the teeth were gently extracted and extreme care was taken to avoid fracture of the socket walls. The tooth was extracted using a #15C blade both mesially and distally to ensure that this was accomplished as atraumatically as possible. The height of the available remaining alveolar bone for implant insertion above the extraction socket apex was estimated by panoramic radiograph whilst accounting for an average X-ray magnification of 30%. The width of the extraction socket was measured with a calibrated periodontal probe intraorally in the mesiodistal and labio-palatal directions.
After thoroughly cleaning the extraction socket with curettes, the implants were placed in the optimal three-dimensional position. At least 3 mm of the implant must be inserted into the apical host bone of the extraction socket to achieve implant primary stability [10]. Existing gap defects were filled with freeze dried bone allograft (FDBA) (Oragraft, LifeNet Health, Virginia Beach, VA, USA). The grafts and implant cover screws were covered with a porcine collagen membrane (lower; Bio-ARM, ACE Surgical Supply Co, Inc., Brockton, MA, USA) and a dPTFE membrane (upper; Cytoplast Regentex GBR-200, Osteogenics Biomedical Inc., Lubbock, TX, USA). The membrane was extended at least 4 to 5 mm onto the intact bony walls of the defect and held securely in place by flap adaptation with monofilament sutures without primary coverage (Fig. 1). Patients were given antibiotics to take three times a day for 7 days. Chlorhexidine (0.12%) oral rinses were also prescribed three times daily for 1 month. The sutures were removed after 10 days.
The upper dPTFE membrane was left exposed for 4 weeks. Wound healing was generally uneventful, showing no signs of infection or other problematic symptoms. The membrane was gently removed using forceps without flap elevation.
A 41-year-old, nonsmoking male patient presented for tooth extraction (tooth #27) and implant placement in the position of teeth #26 and #27. The patient was in good general health. After periodontal and prosthetic evaluation, tooth #27 was gently extracted. Following elevation of a mucoperiosteal flap, implant placement and the GBR procedure were performed using the double-membrane technique. An 11.5-mm-long, 4.1-mm-diameter implant (Osstem SS II, Osstem Implant Co., Seoul, Korea) was placed (Fig. 2A). The coronal gap between the implant and bony walls was filled with FDBA and covered by a collagen membrane and dPTFE membrane (Fig. 2B-D). The wound healing was uneventful. One month after implant placement and GBR, the dPTFE membrane was gently removed without anesthesia (Fig. 3). Two months after implant placement, the cover screw was exposed and the healing cap was connected. The site was then allowed a further healing period of 3 months. A definitive porcelain-fused gold restoration was then placed. Radiographic and clinical evaluation at 16 months postoperatively revealed satisfactory stability and a healthy gingival margin (Fig. 4).
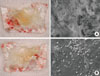
A 30-year-old male patient presented with a history of endodontic complications on tooth #17, which needed to be extracted. The patient had a noncontributory medical history. A 9.5-mm-long, 5.0-mm-diameter implant (SPI CONTACT, Thommen Medical AG, Waldenburg, Switzerland) was immediately placed into the extraction socket following the extraction of tooth #16 and GBR was performed using FDBA, collagen membrane, and dPTFE membrane (double-membrane technique; Fig. 5). The wound healing was uneventful. The upper dPTFE membrane was left exposed for 4 weeks, after which it was gently removed using forceps without flap elevation (Fig. 6). There was considerable plaque deposition on the outer surface of the dPTFE membrane but not on the inner surface. Moreover, the scanning electron microscopy (SEM) of the removed membrane revealed only a small amount of bacteria on the inner surface of the membrane (Fig. 7). The healing cap was connected 3 months after implant placement. Final restorations were delivered after a conventional dental-implant healing period. Clinical and radiological findings revealed favorable peri-implant tissue (Fig. 8).
These cases demonstrate the use of two different membranes in order to overcome the individual disadvantages of resorbable collagen membranes and dPTFE membranes for the GBR procedure. GBR performed utilizing collagen membranes has been widely reported [11,12]. Collagen membranes have several advantageous properties, such as ease of manipulation and good soft-tissue integration, which may be beneficial in clinical applications. Moreover, they do not require a second surgical intervention for their removal, and they have biologically favorable properties including hemostatic, chemostatic, and cell adhesion functions [13]. However, their fast resorption rate, especially on exposure to the oral environment, has raised concerns over their use. Primary soft-tissue closure over the membrane is the most important factor affecting GBR using collagen membranes [11].
Nonporous membranes such as dPTFE show only a small amount of tissue integration, allowing the connective tissue only a minimal degree of attachment to the membrane surface. This may cause unfavorable bone regeneration. However, its minimum pore size (0.2 µm) and surface characteristics do mean that dPTFE has the advantage of preventing bacterial invasion through the membrane [4,14]. On the other hand, a drawback of expanded PTFE (ePTFE) membranes is their surface roughness, which facilitates the adhesion of bacteria. Thus, primary closure over the membrane needs to be achieved to avoid exposure to the oral environment and resulting bacterial colonization.
It would be desirable to combine the advantages of collagen and dPTFE membranes, and the case reports described herein demonstrate the successful use of a collagen membrane (with its optimal behavior toward soft-tissue responses and bone regeneration) as an inner layer and a dPTFE membrane (with its optimal durability and bacterial-protective effect) as an outer layer. There are many reports of successful results of GBR techniques using a barrier membrane in the immediate implantation procedure [15,16]. The inner collagen membrane in this technique prevents connective-tissue down-growth during the healing phase between the socket walls and the implant surface in the most coronal portion of the bone-implant interface, which would prevent osseointegration.
Although primary coverage over the membrane was not obtained in these cases, favorable treatment outcomes were observed in both cases. These outcomes correspond to those observed in previous studies [17-19] in which ePTFE or bioabsorbable membranes were used in addition to grafting material.
The rationale supporting this technique is that a good clinical outcome can be obtained in comparatively unfavorable conditions after uncovering the membrane, due to the outstanding healing potential of a healthy extraction socket.
There are some limitations to using this technique:
1. There should be an intact bony socket wall surrounding an implant that is placed immediately. The presence of a pathologic lesion or bony defect before extraction makes a positive treatment outcome unlikely. In addition, it is important to extend the membrane at least 4 to 5 mm over all remaining bony walls.
2. The top of the implant must be placed at least 1 to 2 mm beneath the top of the surrounding bony wall. This makes the bone regeneration more predictable around the implant due to the favorable osteogenic potential of residual bone and the effect of the bony wall to support a membrane.
3. If a patient is a heavy smoker or has a systemic disease, such as diabetes mellitus, there is a risk of an unfavorable outcome due to a compromised healing capacity.
4. If the oral hygiene condition of a patient is poor, the risk of infection increases.
The use of a grafting material may be helpful in several ways: 1) to prevent collapse of the membrane, especially in the middle section of the extraction socket; 2) to stabilize the blood clot-several studies have found this to be the most important factor in bone regeneration [20]; and 3) although there is some controversy about gap healing around dental implants, we believe that it is beneficial to fill a gap smaller than 2 mm with graft materials to achieve better osseointegration.
On the other hand, we described in our protocol that the membrane removal should be done about 1 month postoperatively. Leaving the membrane in place for a longer duration may increase the risk of complication due to bacterial penetration through the membrane because, although the quantity was small, the bacteria appeared on the inner surface of the dPTFE membrane as shown by SEM (Fig. 7B). Moreover, it was reported that woven bone could be observed already in human biopsy specimens taken in the 2 to 4 week period after extraction due to excellent socket healing [21].
Our clinical cases suggest that successful immediate implant placement is possible using the double-membrane technique when the extraction site is carefully evaluated. Moreover, this implantation method reduces the treatment time when compared with traditional delayed and late implantation methods. It can be concluded that the optimum tissue-integration conditions and biocompatibility of the collagen membrane improve bone regeneration, and the nonporous dPTFE membrane provides protection from the oral environment. Although it was demonstrated that a good clinical outcome can be obtained with this technique, further studies are necessary to evaluate the process in vitro and in vivo, especially with regard to the degree of osseointegration at the crestal area. In addition, histological evaluation and long-term data are needed to confirm the present concept. However, it should be emphasized that the double-membrane technique described in this article is only appropriate for situations where there is no bony defect and where there is a sufficient width of keratinized tissue, since the membrane should be stabilized by a bony wall and a flap around the socket.
The double-membrane technique may be particularly beneficial for immediate implantation in the molar area. In order to reduce the treatment time compared to a two stage approach and avoid displacing the mucogingival junction and performing a second surgical procedure, the implant placement and GBR need to be performed simultaneously and without primary wound closure. This technique provides the clinician with a new treatment modality for immediate implant placement.
Figures and Tables
Figure 1
Schematic drawing showing the appropriate membrane position after immediate implant placement and bone graft. The grafts and implant cover screw were covered with a porcine collagen membrane (lower) and a density polytetrafluoroethylene membrane (upper). It is important to extend the membrane at least 4 to 5 mm over all remaining bony walls and place the top of the implant at least 1 to 2 mm beneath the top of the surrounding bony wall.
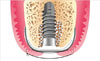
Figure 2
(A) Immediate implant placement after the extraction of tooth #27 (left second molar). (B) Allogenic bone grafting in the coronal gap around the dental implant. (C) Collagen membrane covering the bone-grafted extraction socket and cover screw. (D) Density polytetrafluoroethylene membrane covering the collagen membrane without primary flap closure.
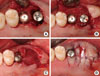
Figure 3
(A) One month after implant surgery. Note the plaque accumulation on the outer surface of the density polytetrafluoroethylene (dPTFE) membrane. (B) Removal of the dPTFE membrane. Note the well-formed soft tissue below the dPTFE membrane.
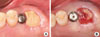
Figure 4
(A) Buccal view of the definitive restoration 16 months postoperatively. (B) Radiographic view 16 months postoperatively. Note the favorable peri-implant soft tissue and the well-maintained crestal bone around the #27 implant.

Figure 5
Placement of a collagen membrane and density polytetrafluoroethylene membrane after immediate implantation (tooth #17, right second molar) and bone grafting.
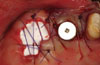
Figure 6
(A) One month after implant surgery. Note the relatively favorable soft-tissue healing without signs of inflammation. (B) Removal of the density polytetrafluoroethylene membrane. Note that the mucogingival junction has not changed after surgery.

Figure 7
Scanning electron microscopy (SEM) view of the density polytetrafluoroethylene membrane after removal. (A) Outer (oral) surface. Note the heavy bacterial deposition on the surface of the membrane. (B) Inner (tissue) surface. Note the small amount of bacteria in the SEM image of the surface (original magnification, ×2,000).
References
1. Covani U, Cornelini R, Barone A. Bucco-lingual bone remodeling around implants placed into immediate extraction sockets: a case series. J Periodontol. 2003. 74:268–273.

2. Paolantonio M, Dolci M, Scarano A, d'Archivio D, di Placido G, Tumini V, et al. Immediate implantation in fresh extraction sockets. A controlled clinical and histological study in man. J Periodontol. 2001. 72:1560–1571.

3. Wilson TG Jr, Schenk R, Buser D, Cochran D. Implants placed in immediate extraction sites: a report of histologic and histometric analyses of human biopsies. Int J Oral Maxillofac Implants. 1998. 13:333–341.
4. Bartee BK. The use of high-density polytetrafluoroethylene membrane to treat osseous defects: clinical reports. Implant Dent. 1995. 4:21–26.
5. Bartee BK, Carr JA. Evaluation of a high-density polytetrafluoroethylene (n-PTFE) membrane as a barrier material to facilitate guided bone regeneration in the rat mandible. J Oral Implantol. 1995. 21:88–95.
6. Crump TB, Rivera-Hidalgo F, Harrison JW, Williams FE, Guo IY. Influence of three membrane types on healing of bone defects. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996. 82:365–374.

7. Walters SP, Greenwell H, Hill M, Drisko C, Pickman K, Scheetz JP. Comparison of porous and non-porous teflon membranes plus a xenograft in the treatment of vertical osseous defects: a clinical reentry study. J Periodontol. 2003. 74:1161–1168.

8. Hoffmann O, Bartee BK, Beaumont C, Kasaj A, Deli G, Zafiropoulos GG. Alveolar bone preservation in extraction sockets using non-resorbabledPTFE membranes: a retrospective non-randomized study. J Periodontol. 2008. 79:1355–1369.

9. Irinakis T. Rationale for socket preservation after extraction of a single-rooted tooth when planning for future implant placement. J Can Dent Assoc. 2006. 72:917–922.
10. Juodzbalys G, Wang HL. Soft and hard tissue assessment of immediate implant placement: a case series. Clin Oral Implants Res. 2007. 18:237–243.

11. Oh TJ, Meraw SJ, Lee EJ, Giannobile WV, Wang HL. Comparative analysis of collagen membranes for the treatment of implant dehiscence defects. Clin Oral Implants Res. 2003. 14:80–90.

12. Moses O, Pitaru S, Artzi Z, Nemcovsky CE. Healing of dehiscence-type defects in implants placed together with different barrier membranes: a comparative clinical study. Clin Oral Implants Res. 2005. 16:210–219.

13. Caffesse RG, Nasjleti CE, Morrison EC, Sanchez R. Guided tissue regeneration: comparison of bioabsorbable and non-bioabsorbable membranes. Histologic and histometric study in dogs. J Periodontol. 1994. 65:583–591.

14. Barber HD, Lignelli J, Smith BM, Bartee BK. Using a dense PTFE membrane without primary closure to achieve bone and tissue regeneration. J Oral Maxillofac Surg. 2007. 65:748–752.

15. Brägger U, Hämmerle CH, Lang NP. Immediate transmucosal implants using the principle of guided tissue regeneration (II). A cross-sectional study comparing the clinical outcome 1 year after immediate to standard implant placement. Clin Oral Implants Res. 1996. 7:268–276.

16. Lang NP, Brägger U, Hämmerle CH, Sutter F. Immediate transmucosal implants using the principle of guided tissue regeneration. I. Rationale, clinical procedures and 30-month results. Clin Oral Implants Res. 1994. 5:154–163.

17. Lekovic V, Camargo PM, Klokkevold PR, Weinlaender M, Kenney EB, Dimitrijevic B, et al. Preservation of alveolar bone in extraction sockets using bioabsorbable membranes. J Periodontol. 1998. 69:1044–1049.

18. Lekovic V, Kenney EB, Weinlaender M, Han T, Klokkevold P, Nedic M, et al. A bone regenerative approach to alveolar ridge maintenance following tooth extraction. Report of 10 cases. J Periodontol. 1997. 68:563–570.

19. Iasella JM, Greenwell H, Miller RL, Hill M, Drisko C, Bohra AA, et al. Ridge preservation with freeze-dried bone allograft and a collagen membrane compared to extraction alone for implant site development: a clinical and histologic study in humans. J Periodontol. 2003. 74:990–999.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download