Abstract
Purpose
The purpose of this study was to compare and quantify the expression of C-reactive protein (CRP), matrix metalloproteinase (MMP)-14, and tissue inhibitor of metalloproteinases (TIMP)-2 in gingival tissues of patients with chronic periodontitis accompanied with inflammatory reaction related to alveolar bone resorption with or without type 2 diabetes mellitus (DM).
Methods
Twelve patients with type 2 DM and chronic periodontitis (group 3), twelve patients with chronic periodontitis (group 2), and twelve healthy individuals (group 1) were included in the study. Gingival tissue biopsies were collected from each patient and from healthy individuals at the time of periodontal surgery (including surgical crown lengthening) or tooth extraction. The concentrations of cytokines were determined by a western blot analysis.
Diabetes mellitus (DM) is the most common metabolic disease worldwide. More than 90% of DM patients have type 2 diabetes [1]. DM is the leading cause of blindness [2], renal failure, and lower limb amputations. DM is a major risk factor for cardiovascular disease, stroke, neuropathy, and periodontitis [1,3].
Chronic periodontitis is the most common type of periodontal disease, and results from extension of the inflammatory process initiated by bacteria in the gingiva to the supporting periodontal tissues. A reciprocal relationship exists between DM and periodontal disease [4]. Periodontal infections, like other infections, have a significant impact on diabetic control.
Conversely, DM is a significant risk factor for the development of periodontal disease and aggravates the severity of periodontal infections [5]. The comorbidity of these two inflammatory diseases suggests that there are common elements of pathogenesis related to the risks for both conditions.
In patients with periodontal disease, chronic low-level systemic exposure to periodontal microorganisms may occur, leading to significant changes in the plasma levels of cytokines and hormones. Due to the dynamic nature of the inflamed periodontium, the tissue may serve as an endocrine-like source of inflammatory mediators. Among the inflammatory biomarkers examined, C-reactive protein (CRP), and Interleukin (IL)-6 appear to be involved, according to plausible biological mechanisms through which they function, examined in studies of the links between periodontal disease and cardiovascular disease [5]. Recently, Blüher et al. [6] investigated whether the plasma concentrations of inflammatory markers were associated with measures of obesity, insulin sensitivity, and hyperglycemia, and discovered significant correlations between the plasma concentrations of all of the inflammatory markers examined and percent body fat, insulin sensitivity, and fasting plasma glucose. Fasting plasma glucose was a significant determinant of adiponectin, CRP, and IL-6 plasma concentrations, whereas body fat content was a significant predictor only of CRP plasma concentration [6]. In a similar study, the authors concluded that type 2 DM was highest among subjects with elevated levels of IL-18 and CRP or IL-18 and IL-6.
CRP is an acute-phase reactant synthesized by the liver in response to inflammatory cytokines, IL-6, IL-1, and tumor necrosis factor-alpha (TNF-α). Circulating CRP levels are a marker of systemic inflammation and are associated with periodontal disease [7], a chronic bacterial infection associated with the elevation of proinflammatory cytokines and prostaglandin [8]. Elevated immunoglobulin G induced by bacterial species associated with destructive periodontal diseases is associated with the increase in CRP [9]. Standard non-surgical periodontal therapy resulted in a decrease in serum CRP levels [10] in a previous study.
Microbial components, especially lipopolysaccharide, activate macrophages to synthesize and secrete a variety of proinflammatory molecules, including the cytokines IL-1 and TNF-α; prostaglandins, especially prostaglandin E2; and hydrolytic enzymes. Similarly, bacterial substances activate T lymphocytes to produce IL-1 and lymphotoxin, a molecule with similar properties to TNF-α. These cytokines manifest potent proinflammatory and catabolic activities, and play key roles in periodontal tissue breakdown through collagenolytic enzymes such as matrix metalloproteinases (MMPs) [11].
The MMPs are a family of structurally and functionally related enzymes that are responsible for the proteolytic degradation of extracellular matrix components. More than 20 different MMPs have been identified. These proteins can be classified into the following subgroups based on their substrate specificities and structural homologies: collagenase (MMP-1, -8, and -13), gelatinase (MMP-2, and -9), stromelysin (MMP-3, -10, and -11), membrane-type MMPs (MT-MMPs- : MMP-14, 15, -16, -17, -23, -24, and -25) and other MMPs, including matrilysin (MMP-7) and metalloelastase (MMP-12) [12].
Expression of MMPs is low in normal cells, and these low levels allow for healthy connective tissue remodeling. In pathologic conditions, however, the level of MMP expression increases considerably, resulting in aberrant connective tissue destruction. Excess MMP production is associated with the pathology of many diseases, including periodontitis [13], atherosclerosis [14], tumor invasion/metastasis, and arthritic disease [15].
MT-MMPs are a unique class of MMPs anchored to the cell surface by transmembrane domains. MT-MMPs display a broad spectrum of activities, and because of their localization to the cell surface, they are thought to play a major role in controlling proteolytic events within the pericellular microenvironment. MT1-MMP (MMP-14), can degrade a variety of extracellular matrix proteins and, is capable of activating both pro-MMP-2 and pro-MMP-13 [16].
MMP-17 and 25 are glycosylphosphatidylinositol-anchored whereas the other four MT-MMPs are type 1 transmembrane proteins with short cytoplasmic domains of about 20 amino acids. Like secreted MMPs, MT-MMPs can cleave extracellular matrix molecules, as well as chemokines, cytokines, and growth factors [17]. MT-MMPs are generally thought to play important regulatory roles because of their ability to cleave substrates in the immediate vicinity of the cell membrane, where the cleaved products can interact with cell-surface receptors. In addition, MT-MMPs are known to cleave and activate secreted MMPs, which were first described in the case of activation of MMP-2 by MMP-14 through interaction with TIMP-2.
MMPs are regulated at several levels including transcription, secretion, activation, and inhibition. Regulation by the latest of these mechanisms is via endogenous inhibitors, known as TIMPs. The balance between the levels of active enzymes and free TIMPs is thought to determine overall MMP activity. The TIMPs are small proteins (-23kDa) that inhibit MMP activity by binding to them in a 1:1 stoichiometric ratio [18]. The four members of the TIMP family (TIMP-1 through TIMP-4) are cysteine-rich proteins stabilized by disulphide bonds [19]. They are composed of a large N-terminal domain responsible for MMP inhibition and a smaller C-terminal domain. The TIMPs in general do not demonstrate specificity for any particular MMP [18], although TIMP-2 shows some degree of preference for MMP-2 and TIMP-1 for MMP-9 [20].
TIMP-2 plays a role not only in MMP inhibition, but also MMP activation. The relative levels of TIMP-2 and MMP-14 are critical, since low TIMP-2 levels are associated with MMP-14 mediated activation of pro-MMP-2 and at higher TIMP-2 levels MT1-MMP function is blocked, preventing pro-MMP-2 activation [21].
The particular roles and substrate specificities of MT-MMPs and TIMP-2 have not been described in detail, and the role of MT-MMPs and TIMP-2 in DM is unknown.
In inflammatory responses with bone resorption, the roles and interactions of CRP, MMP-14, and TIMP-2 are not clear. Their contribution to the pathogenesis of periodontitis and alveolar bone resorption has not yet been established. Moreover, no in vivo studies have simultaneously analyzed CRP, MMP-14, and TIMP-2, and their interrelationship in diabetic and nondiabetic patients with chronic periodontitis. The purpose of this study was to compare and quantify the expressions of CRP, MMP-14, and TIMP-2, in gingival tissues in order to reveal diagnostic factors in chronic periodontitis patients with or without type 2 DM.
The study population consisted of 12 patients with type 2 DM and chronic periodontitis, 12 patients with chronic periodontitis, and 12 healthy individuals. Marginal gingival tissue samples were obtained in the Department of Periodontology, Kyungpook National University Hospital, Korea, by internal bevel incision at the time of periodontal surgery (including surgical crown lengthening) or tooth extraction. All participants signed the Institutional Review Board-approved (No. 74005-418) consent form prior to surgery.
According to the patient's systemic condition (age, sex, blood glucose level, obesity, and smoking), the clinical criteria of the gingiva (sulcus bleeding index value and probing depths), and radiographic evidence of bone resorption, each gingival sample was assigned to one of three groups. Group 1 (normal, n=12) consisted of clinically healthy gingiva without bleeding, evidence of bone resorption, or periodontal pockets, obtained from 12 systemically healthy patients. Group 2 (chronic periodontitis, n=12) consisted of the inflamed gingiva of patients with chronic periodontitis. The diagnosis of chronic periodontitis was established on the basis of clinical and radiographic criteria (bone resorption) according to the classification system for periodontal disease and conditions. All patients in group 2 were systemically healthy and had more than one periodontal pockets ≥5 mm and at least one pocket with ≥5 mm loss of attachment. All gingival samples were obtained from teeth with a probing depth ≥5 mm, swelling of the marginal gingiva, and bleeding corresponding to gingival sulcus bleeding index 3 according to Mühlemann and Son [22]. Group 3 (chronic periodontitis and type 2 DM, n=12) consisted of the inflamed gingiva of patients with chronic periodontitis associated with type 2 DM. Patients in group 3 were diagnosed with type 2 DM at least 6 months prior and showed blood glucose levels of 200 mg/dL and above in the first postprandial 2 hours. Patients in groups 2 and 3 had similar periodontal conditions, but patients in group 2 were systemically healthy and patients in group 3 had type 2 DM. Gingival samples were obtained as described above. Patient characteristics are presented in Table 1.
Following surgery, excised tissue specimens were immediately frozen in liquid nitrogen at -70℃.
The western blotting, technique has been as previously described by Park and Lee [13]. Frozen tissues were homogenized in radio-immunoprecipitation assay lysis buffer (10 mM ethylenediaminetetraacetic acid, 0.15 M NaCl) with 1:30 diluted protease inhibitor cocktail (Roche, Mannheim, Germany). The lysates were sonicated three times for 10 seconds and centrifuged at 12,000 g for 20 minutes. Protein concentrations of the supernatant were routinely determined by a Braford protein asssay (Quick Start, BIO-rad Laboratories Inc., Hercules, CA, USA) using bovine serum albumin (BSA) as standard.
Lysates were boiled in a sodium dodecyl sulfate (SDS) sample buffer (1 M Tris-HCl [pH 6.8], 40% glycerol, 8% SDS, 2% mercapto-ethanol, 0.002% Bromophenol blue). Prepared samples were separated by 15% SDS-polyacrylamide gels and transferred to a polyvinylidene difluoride membrane.
The membranes were subsequently blocked in tris-buffered saline (TBS) containing 5% powdered milk and 1% BSA for 1 hour, and then incubated with polyclonal anti-CRP antibody, anti-MMP-14 antibody, and anti-TIMP-2 antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) for 1.5 hours at room temperature.
The membranes were washed (five times for 5 minutes with Tween 20) and incubated with a horseradish peroxidase-conjugated goat anti-rabbit secondary antibody for anti-CRP antibody, anti-MMP-14 antibody, and anti-TIMP-2 antibody (diluted 1:2,000 in TBS) for 1 hour at room temperature. After additional washing (five times for 5 minutes with Tween 20) the western blot procedure was completed with an ECL Plus development kit (Amsterdam, Beckinghamshire, UK).
The relative quantification analysis of CRP, MMP-14, and TIMP-2 expression was performed using a densitometer (Image Gauge V 3.46, Fuji Photo Film Co., Tokyo, Japan). After normalization to β-actin (Abcam plc, Cambridge, UK) in each sample, levels of CRP, MMP-14, and TIMP-2 were expressed as a ratio of CRP, MMP-14, or TIMP-2 to β-actin and the differences in density between the 3 groups were determined.
All data were presented as means±standard deviation and results were statistically analyzed. The CRP, MMP-14, and TIMP-2 levels were compared using a one-way analysis of vaiance followed by Tukey's test. P-value <0.05 was considered to statistically significant.
Representative western blot data comparing CRP expression levels in human gingiva with chronic periodontitis with or without type 2 DM are presented in Fig. 1. The comparison of CRP expression levels were also studied by detecting a CRP band with a molecular weight of about 27 kDa and measuring their density and areas in all three groups (Fig. 1). The comparative levels of normalized CRP expression are given in Fig. 2. The mean value of CRP expression (ratio of CRP/β-actin) was 0.669±0.119 for group 1, 0.679±0.134 for group 2, and 0.859±0.186 for group 3. There were statistically significant differences between group 1 and group 3 and between group 2 and group 3, but there was no statistically significant difference (P<0.05) between group 1 and group 2. The comparison of MMP-14 expression levels was also performed by western blot analysis using MMP-14 specific antibody which detected MMP-14 in all three groups (Fig. 3). The comparative levels of MMP-14 expression were also quantified with β-actin normalization (Fig. 4). The mean value of MMP-14 expression (ratio of MMP-14/β-actin) was 0.542±0.104 for group 1, 0.706±0.133 for group 2, and 0.954±0.248 for group 3. There were statistically significant differences between group 1 and group 2 (P<0.05) and between group 1 and group 3 (P<0.05). In this study, the molecular weight of TIMP-2 was identified to be 28 kDa in western blot analysis (Fig. 5). The comparative levels of TIMP-2 expression were also quantified with β-actin normalization and presented in Fig. 6. The mean value of TIMP-2 expression (ratio of TIMP-2/β-actin) was 0.485±0.098 for group 1, 0.605±0.084 for group 2, and 0.746±0.181 for group 3. There were statistically significant differences between group 1 and group 3 and between group 2 and group 3 (P<0.05).
Among the many oral problems that can occur because of diabetes, the prevalence of severe periodontitis is significantly higher among people with poorly controlled DM [23], to the extent that periodontitis has been called the "sixth complication of diabetes" [24]. The reason for the higher rates of periodontal disease in people with diabetes is not completely understood, but studies have reported that there is little difference in the periodontal flora of people with and without DM, and suggest that the increased destruction of tissue among those with diabetes may be due to an altered host susceptibility to periodontal pathogens mediated by the accumulation of advanced glycation end products in the tissues, microvascular changes, and perhaps impaired lipid metabolism [25]. Conversely, these data also suggest that the presence of periodontal infection can adversely affect glycemic control in people with diabetes and that there appears to be a bi-directional relationship between the two conditions.
The purpose of this study was to quantify and compare the expression of CRP, MMP-14 and TIMP-2 in the gingival tissues of patients with chronic periodontitis associated to type 2 DM, in order to understand the diagnostic contribution of these proteins to periodontal destruction accompanied with alveolar bone resorption in type 2 diabetic patients.
CRP is an acute-phase protein suggesting a central role in immunological response [26]. It is synthesized in the liver mainly in response to IL-6 and binds to the polysaccharides of pathogens promoting phagocytosis [27]. Several studies have shown that CRP could be useful in infection diagnosis [28], as well as in monitoring the response to antibiotic therapy [29].
In this study, the quantitative analysis of CRP levels showed that CRP expression increased significantly in inflamed gingiva with or without type 2 DM compared to healthy gingiva. Differences were statistically significant between group 1 and group 3 and between group 2 and group 3 (P<0.05) (Table 2, Fig. 2).
Our results are similar to previous studies in which Noack et al. [7] and Craig et al. [9] found that subjects with periodontal disease demonstrated higher levels of CRP than subjects without periodontitis.
We found a statistically significant difference between periodontitis subjects with DM (group 3) and subjects with periodontitis only (group 2). The ongoing acute phase response (seen in insulin-resistant subjects and type II DM patients) is induced by cytokines, and is reflected in elevated circulating inflammatory markers, such as CRP, IL-1, IL-6, TNF-α, leptin, plasminogen activator inhibitor-1, angiotensinogen, and fibrinogen, as described by Hsueh and Bruemmer [30]. It seems that the increased levels of CRP were influenced by DM during the progression of inflammation.
Microbial components, especially lipopolysaccharides, activate macrophages to synthesize and secrete a variety of proinflammatory molecules, including the cytokines IL-1 and TNF-alpha, prostaglandins, especially prostaglandin E2, and hydrolytic enzymes. Similarly, bacterial substances activate T lymphocytes to produce IL-1 and lymphotoxin, a molecule with similar properties to TNF-alpha. These cytokines manifest potent proinflammatory and catabolic activities, and play key roles in periodontal tissue breakdown through collagenolytic enzymes such as MMPs.
MMP-14 was initially identified as the activator of MMP-2, but has recently been shown to be important for wound healing, angiogenesis, and inflammation [31]. It is over-expressed in cancers leading to migration, invasion, and metastasis [32]. MMP-14 activation occurs via a proprotein convertase, and recent data suggests that its function is modified by glycosylation, internalization and recycling [33]. Finally MMP-14 directly degrades extracellular matrix molecules, including collagen type I, III, laminin, and fibronectin [34].
In this study, the quantitative analysis of MMP-14 levels showed that MMP-14 expression was rather increased in inflamed gingiva with or without type 2 DM compared to healthy gingiva. The differences were statistically significant between group 1 and group 2 and between group 1 and group 3 (P<0.05) (Table 3, Fig. 4).
Song and Ergul [35] reported that mild elevation of blood glucose for 6 weeks is sufficient to stimulate gene expression of MMP-2, MMP-9, and MMP-14.
The MMP-14 level in group 3 was higher than group 2, but difference was not statistically significant. It can be assumed that this is a result of a higher inflammatory response in chronic periodontitis associated with type 2 DM.
A major group of inhibitors of the MMPs, TIMP-2, has been studied in this study. The levels of TIMP-2 were significantly different between group 1 and 3 and between group 2 and group 3 (P<0.05) (Table 4, Fig. 6). However, there has been some debate over findings regarding TIMP-2 in diabetic subjects. MecLennan et al. [36] observed no changes in TIMP-2 expression in mesangial cells cultured in high glucose. Our results also show increased TIMP-2 levels. It can be assumed that this is a result of a greater inflammatory response in chronic periodontitis associated with type 2 DM.
In conclusion, increased levels of CRP, MMP-14, and TIMP-2 are useful for diagnosing and monitoring inflammatory disease that includes moderate periodontitis.
More studies are needed to investigate interrelationship among CRP, MMP-14, TIMP-2, and other factors that affect the progression of periodontal disease to a more advanced level.
Figures and Tables
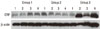 | Figure 1C-reactive protein (CRP) western analysis showing 4 representative blots in each group. C-reactive protein levels were quantified on the basis of β-actin levels. CRP corresponding to a molecular weight of 27 kDa was expressed in all groups, including healthy gingiva, and the expression levels of C-reactive protein increased in order from group 1 to group 2 to group 3. Group 1, healthy gingiva from systemically healthy persons; Group 2, inflamed gingiva from patients with chronic periodontitis; Group 3, inflamed gingiva from patients with chronic periodontitis and type 2 diabetes mellitus. |
 | Figure 2C-reactive protein (CRP) levels (ratio of CRP/β-actin) in groups 1, 2, and 3. In inflamed gingiva with diabetes (group 3), the levels of CRP were significantly increased as compared to group 1 and group 2 (P<0.05). a)Significant difference between group 1 and group 3 (P<0.05). b)Significant difference between group 2 and group 3 (P<0.05). |
 | Figure 3Matrix metalloproteinase (MMP)-14 western analysis showing 4 representative blots in each group. MMP-14 levels were quantified on the basis of β-actin levels. MMP-14 corresponding to a molecular weight of 60 kDa was shown to be expressed in all groups including healthy gingiva. The expression levels of Matrix metalloproteinase 14 increased in order from group 1 to group 2 to group 3. Group 1, healthy gingiva from systemically healthy persons; Group 2, inflamed gingiva from patients with chronic periodontitis; Group 3, inflamed gingiva from patients with chronic periodontitis and type 2 diabetes mellitus. |
 | Figure 4Matrix metalloproteinase (MMP)-14 levels (ratio of MMP-14 to β-actin) in groups 1, 2, and 3. In the inflamed gingiva (with or without diabetes, group 3 and group 2, respectively), the levels of MMP-14 were higher than those of healthy gingiva. a)Significant difference between group 1 and group 2 (P<0.05). b)Significant difference between group 1 and group 3 (P<0.05). |
 | Figure 5Tissue inhibitor of metalloproteinase (TIMP)-2 western analysis showing 4 representative blots in each group. TIMP-2 levels were quantified on the basis of β-actin levels. TIMP-2 corresponding to a molecular weight of 28 kDa was expressed in all samples including healthy gingiva. The expression levels of TIMP-2 were increased in patients with type 2 diabetes mellitus compared to healthy control subjects. Group 1, healthy gingiva from systemically healthy persons; Group 2, inflamed gingiva from patients with chronic periodontitis; Group 3, inflamed gingiva from patients with chronic periodontitis and type 2 diabetes mellitus. |
 | Figure 6Tissue inhibitor of metalloproteinases (TIMP)-2 level (Ratio of TIMP-2 to β-actin) in groups 1, 2, and 3. In the inflamed gingiva (with or without diabetes, groups 3 and 2, respectively), the levels of TIMP-2 were higher than those of healthy gingiva (P<0.05). a)Significant difference between group 1 and group 2 (P<0.05). b)Significant difference between group 2 and group 3 (P<0.05). |
References
1. Engelgau MM, Geiss LS, Saaddine JB, Boyle JP, Benjamin SM, Gregg EW, et al. The evolving diabetes burden in the United States. Ann Intern Med. 2004. 140:945–950.

2. Fong DS, Aiello L, Gardner TW, King GL, Blankenship G, Cavallerano JD, et al. Diabetic retinopathy. Diabetes Care. 2003. 26:226–229.

3. Graves DT, Liu R, Alikhani M, Al-Mashat H, Trackman PC. Diabetes-enhanced inflammation and apoptosis: impact on periodontal pathology. J Dent Res. 2006. 85:15–21.

4. Committee on Research, Science and Therapy. American Academy of Periodontology. Diabetes and periodontal diseases. J Periodontol. 2000. 71:664–678.
5. Pradhan AD, Ridker PM. Do atherosclerosis and type 2 diabetes share a common inflammatory basis? Eur Heart J. 2002. 23:831–834.

6. Blüher M, Fasshauer M, Tönjes A, Kratzsch J, Schön MR, Paschke R. Association of interleukin-6, C-reactive protein, interleukin-10 and adiponectin plasma concentrations with measures of obesity, insulin sensitivity and glucose metabolism. Exp Clin Endocrinol Diabetes. 2005. 113:534–537.

7. Noack B, Genco RJ, Trevisan M, Grossi S, Zambon JJ, De Nardin E. Periodontal infections contribute to elevated systemic C-reactive protein level. J Periodontol. 2001. 72:1221–1227.

8. Page RC. The role of inflammatory mediators in the pathogenesis of periodontal disease. J Periodontal Res. 1991. 26(3 Pt 2):230–242.

9. Craig RG, Yip JK, So MK, Boylan RJ, Socransky SS, Haffajee AD. Relationship of destructive periodontal disease to the acute-phase response. J Periodontol. 2003. 74:1007–1016.

10. D'Aiuto F, Ready D, Tonetti MS. Periodontal disease and C-reactive protein-associated cardiovascular risk. J Periodontal Res. 2004. 39:236–241.
11. Sorsa T, Ingman T, Suomalainen K, Haapasalo M, Konttinen YT, Lindy O, et al. Identification of proteases from periodontopathogenic bacteria as activators of latent human neutrophil and fibroblast-type interstitial collagenases. Infect Immun. 1992. 60:4491–4495.

12. Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001. 17:463–516.

13. Park JW, Lee JM. The comparison of IL-6, elastase and alpha1-PI expressions in human chronic periodontitis with type 2 diabetes mellitus. J Korean Acad Periodontol. 2007. 37:Suppl. 325–338.

14. Borden P, Heller RA. Transcriptional control of matrix metalloproteinases and the tissue inhibitors of matrix metalloproteinases. Crit Rev Eukaryot Gene Expr. 1997. 7:159–178.

15. Brinckerhoff CE, Rutter JL, Benbow U. Interstitial collagenases as markers of tumor progression. Clin Cancer Res. 2000. 6:4823–4830.
16. Itoh Y, Seiki M. MT1-MMP: a potent modifier of pericellular microenvironment. J Cell Physiol. 2006. 206:1–8.

17. Stamenkovic I. Extracellular matrix remodelling: the role of matrix metalloproteinases. J Pathol. 2003. 200:448–464.

18. Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta. 2000. 1477:267–283.

19. Williamson RA, Marston FA, Angal S, Koklitis P, Panico M, Morris HR, et al. Disulphide bond assignment in human tissue inhibitor of metalloproteinases (TIMP). Biochem J. 1990. 268:267–274.

20. Goldberg GI, Strongin A, Collier IE, Genrich LT, Marmer BL. Interaction of 92-kDa type IV collagenase with the tissue inhibitor of metalloproteinases prevents dimerization, complex formation with interstitial collagenase, and activation of the proenzyme with stromelysin. J Biol Chem. 1992. 267:4583–4591.

21. Butler GS, Butler MJ, Atkinson SJ, Will H, Tamura T, Schade van Westrum S, et al. The TIMP2 membrane type 1 metalloproteinase "receptor" regulates the concentration and efficient activation of progelatinase A. A kinetic study. J Biol Chem. 1998. 273:871–880.

22. Mühlemann HR, Son S. Gingival sulcus bleeding: a leading symptom in initial gingivitis. Helv Odontol Acta. 1971. 15:107–113.
23. Cho JY, Xing S, Liu X, Buckwalter TL, Hwa L, Sferra TJ, et al. Expression and activity of human Na+/I- symporter in human glioma cells by adenovirus-mediated gene delivery. Gene Ther. 2000. 7:740–749.

24. Tsai C, Hayes C, Taylor GW. Glycemic control of type 2 diabetes and severe periodontal disease in the US adult population. Community Dent Oral Epidemiol. 2002. 30:182–192.

25. Cutler CW, Shinedling EA, Nunn M, Jotwani R, Kim BO, Nares S, et al. Association between periodontitis and hyperlipidemia: cause or effect? J Periodontol. 1999. 70:1429–1434.

26. Vigushin DM, Pepys MB, Hawkins PN. Metabolic and scintigraphic studies of radioiodinated human C-reactive protein in health and disease. J Clin Invest. 1993. 91:1351–1357.

27. Mold C, Gewurz H, Du Clos TW. Regulation of complement activation by C-reactive protein. Immunopharmacology. 1999. 42:23–30.

28. Ugarte H, Silva E, Mercan D, De Mendonça A, Vincent JL. Procalcitonin used as a marker of infection in the intensive care unit. Crit Care Med. 1999. 27:498–504.

29. Póvoa P, Coelho L, Almeida E, Fernandes A, Mealha R, Moreira P, et al. C-reactive protein as a marker of ventilator-associated pneumonia resolution: a pilot study. Eur Respir J. 2005. 25:804–812.

30. Hsueh WA, Bruemmer D. Peroxisome proliferator-activated receptor gamma: implications for cardiovascular disease. Hypertension. 2004. 43:297–305.

31. Zucker S, Pei D, Cao J, Lopez-Otin C. Membrane type-matrix metalloproteinases (MT-MMP). Curr Top Dev Biol. 2003. 54:1–74.

32. Seiki M, Yana I. Roles of pericellular proteolysis by membrane type-1 matrix metalloproteinase in cancer invasion and angiogenesis. Cancer Sci. 2003. 94:569–574.

33. Wu YI, Munshi HG, Sen R, Snipas SJ, Salvesen GS, Fridman R, et al. Glycosylation broadens the substrate profile of membrane type 1 matrix metalloproteinase. J Biol Chem. 2004. 279:8278–8289.

34. Itoh Y, Seiki M. MT1-MMP: an enzyme with multidimensional regulation. Trends Biochem Sci. 2004. 29:285–289.





 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download