Abstract
Purpose
The present study was performed to clarify the relationship between periodontal disease severity and selected immunological parameters consisting of serum IgG titer against periodontopathogenic bacteria, the expression of the helper T-cell cytokine by gingival mononuclear cells, and patients' immunoreactivity to cross-reactive heat shock protein (HSP) epitope peptide from P. gingivalis HSP60.
Methods
Twenty-five patients with moderate periodontitis had their gingival connective tissue harvested of gingival mononuclear cells during an open flap debridement procedure and peripheral blood was drawn by venipuncture to collect serum. The mean level of interproximal alveolar bone was calculated to be used as an index for periodontal disease severity for a given patient. Each of selected immunologic parameters was subject to statistical management to seek their correlations with the severity of periodontal disease.
Results
A significant correlation could not be identified between serum IgG titers against specific bacteria (Porphyromonas gingivalis, Prevotella intermedia, Fusobacterium nucleatum, Actinobacillus actinomycetemcomitans, and Streptococcus mutans) and the severity of periodontal disease. Expression of interleukin (IL)-10 by gingival mononuclear cells was statistically significant in the group of patients who had higher levels of alveolar bone height. However, a similar correlation could not be demonstrated in cases for IL-4 or interferon-γ. Patients' serum reactivity to cross-reactive epitope peptide showed a significant correlation with the amount of alveolar bone.
Periodontal disease, initiated and perpetuated by concerted interaction between host inflammatory and immunological responses to the specific infecting periodontopathogenic bacteria, is characterized by destruction and exacerbation of gingival connective tissue and alveolar bone leading to eventual loss of teeth [1,2].
Earlier studies have focused on the infectious nature of disease caused by polymicrobial infection, while other studies have reported the relevance of disease severity to the serum antibody titer to an array of specific bacteria [3-5]. Most of the bacteria that cause the periodontal disease are gram-negative anaerobic or aerobic bacteria including Porphyromonas gingivalis, Actinobacillus actinomycetemcomitans, Prevotella intermedia, and Fusobacterium nucleatum [5,6]. Naito et al. [4] reported that the serum antibody titer against P. gingivalis was significantly higher in patients with periodontal disease than in healthy controls, and found that the high antibody titer was remarkably reduced after periodontal treatment [7]. Kojima et al. [8] also reported a close positive correlation between the serum antibody titer against P. gingivalis and periodontal disease status. According to the longitudinal study performed by Taubman et al. [9], the serum antibody to a specific oral bacterium remained comparatively stable; however, the titer reflected the disease activity, i.e. active or quiescent. It appears that the antibody response to bacteria may play a critical role in the host defense system, but there have not been any clear explanations with regard to the functional role of the serum antibody.
The latest concept based in osteoimmunology claims that the activated T-cell is the primary source of the receptor activator for nuclear factor kappa B ligand (RANKL), which is necessary for the differentiation and activation of osteoclasts stimulating alveolar bone destruction. Based on a variety of studies carried out on the bacterial antigen-specific CD4-positive T-cell secreting characteristic cytokine profiles, the type 1 helper T-cell is reported to be closely related to the expression of RANKL-mediated alveolar bone destruction [10,11]. In contrast, a type 2 helper T-cell cytokine, interleukin (IL)-10, is known to inhibit bone resorption as reported by Liu et al. [12], who observed that IL-10 promotes the expression of osteoprotegerin and inhibits the expression of colony stimulating factor-1 and RANKL. This hypothesis is supported by the observation that alveolar bone destruction was pronounced in IL-10 knockout mice in experimentally-induced periodontitis [13-15]. Takayanagi et al. [16] reported that the type 1 helper T-cell cytokine, interferon gamma (IFN-γ), disturbs the RANKL-RANK signaling system and inhibits alveolar bone destruction. However, it turned out to promote the expression of RANKL, thus increasing the alveolar bone destruction [17-19].
As the autoimmune characteristics of periodontal disease have recently been demonstrated, heat shock protein (HSP), a principal antigen that stimulates the host immunological response, has attracted scientific interest. It is generated when cells are exposed to various stimuli and is well conserved through evolution maintaining high degree of sequence homology between mammalian and bacterial HSPs [20,21]. Choi et al. [22,23] and Chung et al. [24] have demonstrated the immunologic cross-reactivity of HSP in the context of provoking autoimmune diseases such as arteriosclerosis and rheumatoid arthritis. Ueki et al. [25] found the cross-reaction between periodontal pathogenic bacteria and human HSP, which stimulated the expression of pro-inflammatory cytokine and induced chronic tissue destruction from the periodontal lesion. Nevertheless, most recent studies claim that T-cells specific to human HSP manifest the immunoregulatory phenotype characteristic of CD4, CD25, and FoxP3 phenotypes, and functions to inhibit autoimmune responses [26,27]. The stimulus to bacterial HSP60-specific epitope, which cross-reacts with human HSP60, activated the regulatory T-cell in experiments with arthritic Lewis rats [28-30]. Lee et al. [31] have recently identified a peptide epitope (TLVVNRLRGSLKICAVKAPG) of P. gingivalis HSP60 that shows cross-reactivity with its human counterpart with the prospect of defining immunoregulatory function of the epitope. While several studies have been carried out as to what relationship bacterial HSP60 has to arteriosclerosis or rheumatic arthritis, there have been few studies on the exact role of bacterial HSP60 in the etiology of periodontal disease.
The question arises as to what kinds of immunologic parameters, either alone or in combination, are closely associated with periodontal disease severity. Hence, the present study was performed to clarify the relationship between periodontal disease severity and an array of immunological parameters consisting of the expression of the helper T-cell cytokine, the serologic reactivity to cross-reactive HSP peptide, and serum immunoglobulin G (IgG) titer against periodontal pathogenic bacteria.
Twenty-five patients (12 males and 13 females, 39-63 years of age), who were diagnosed as having moderate chronic periodontitis at the Department of Periodontics Clinic, Pusan National University Hospital, participated in the study. They had no history of systemic diseases contributing to periodontal disease. Prior to treatment, a 5-mL sample of blood was collected by venipuncture. Gingival connective tissue samples were harvested via open flap debridement to collect gingival mononuclear cells. The level of alveolar bone was evaluated on the basis of alveolar bone height, being calculated as a proportion (%) of the relative distance between the alveolar crest and root apex to the total length of the root measured on the radiographic image. For defining the periodontal disease severity, patients were classified into two groups; one group (Group A) consisted of those whose alveolar bone heights were greater than 70%, while the other group (Group B) consisted of those with bone heights under 70%. This study was approved by the Pusan National University Dental Hospital Institutional Review Board (2008019).
Serum IgG titer to periodontopathogenic bacteria were determined by enzyme-linked immunosorbent assay (ELISA). Briefly, microtiter plates were coated respectively with bacteria (P. ginivalis, A. actinomycetemcomitans, P. intermedia, F. nucleatum, Streptococcus mutans) and diluted (10 µg/mL) in phosphate buffer [2]. The plates were washed and an aliquot of serum samples serially diluted was added and incubated. The plates were washed, and peroxidase-conjugated mouse antihuman IgG (γ-chain-specific, Jackson ImmunoResearch Laboratories, West Grove, USA) was added. After 2 hours of incubation, the plates were washed and an aliquot of tetramethylbenzidine (Kirkegaard and Perry Laboratories, Gaithersburg, USA) was added for incubation followed by the addition of 0.18 M H2SO4 to stop the reaction. Optical densities read at 450 nm were plotted as a function of the serum dilution factor. The serum dilution factor corresponding to an optical density of 0.5 was designated as antibody titer.
Total RNA was isolated from gingival tissues using the Trizol method and dried until solubilization for reverase transcriptase-polymerase chain reaction (RT-PCR). For detection of gene expression, 2 µg of total RNA from each sample was reverse transcribed by M-MLV reverse transcriptase (Invitrogen, Carsbad, USA) to synthesize cDNA, in a 25 µL reaction volume, according to the manufacturer's instructions. The reverse transcription reaction was carried out at 37℃ for 1 hour, followed by a 10-minute incubation at 70℃. Primers were designed based on the cDNA nucleotide sequences of the human IL-4 gene, IL-10 gene, IFN-γ gene, and β-actin. Oligonucleotide primers are as follows: IL-4, 5'-AGAGCAGAAGACTCTGTGCA- 3' (sense) and 5'-CCAACGTACTCTGGTTGGCT-3' (antisense); IL-10, 5'-CCAACGTACTCTGGTTGGCT-3' (sense) and 5'-GCCTTTCTCTT GGAGCTTAT-3' (antisense); IFN-γ, 5'-CAGGACCCATATGTAAAAGA-3' (sense) and 5'-TCGCTTCCCTGTTTTAGCTG-3' (antisense); and β-actin, 5'-TAAGGAGAAGCTGTGCTACG-3' (sense) and 5'-CCGATCCACACGGAGTACTT-3' (antisense). Polymerase chain reaction was performed in a 100 µL reaction volume containing 10 µL of first-strand cDNA as a template, 10X PCR reaction buffer, 2 µL of each dNTP, 0.5 µL of each sense primer, 0.3 µL of each antisense primer, and 0.5 µL of ampli-Taq DNA polymerase (Applied Biosystems, Foster City, USA). The reaction was carried out for 35 cycles. For each cycle, denaturation was carried out at 94℃ for 1 minute, annealing at 49-59℃ for 1 minute, and extension at 72℃ for 1 minute. The β-actin gene was amplified in parallel, in all PCR amplifications, as an internal control. After the PCR reaction, 10 µL of PCR product was loaded onto an 1.7% agarose gel and stained with ethidium bromide.
Peptides #19 of P. gingivalis HSP60 (TLVVNRLRGSLKICAVKAPG) and human HSP60 (TLVLNRLKVGLQVVAVKAPG) were each spotted onto polyvinyliden fluoride membrane. The membrane was blocked with 5% skim milk followed by adding the monoclonal antibody in PBS buffer for incubation for 2 hours at room temperature. After washing the membrane, horseradish peroxidase-conjugated goat anti-mouse IgG (γ-chain specific, Jackson ImmunoResearch Laboratories, West Grove, USA) was added and incubated for 1 hour. The membrane was then washed with PBS-Tween followed by adding tetramethylbenzidine for color development.
For statistical processing, SPSS ver. 14.0 (SPSS Inc., Chicago, USA) was used. The significance level was determined at a 95% confidence interval. To compare the serum antibody titers of periodontal pathogenic bacteria according to alveolar bone levels, the independent samples t-test was applied to the average calculated in each group. The correlation between the expression patterns of helper T-cell cytokine, related to alveolar bone level, and the serologic reactivity of cross-reactive HSP peptide, was analyzed by use of Fisher's exact test.
An open flap debridement procedure was performed on 25 patients with chronic periodontitis, and gingival connective tissues were harvested in the process of surgery. According to the criteria described in the methods section, all the patients were classified into two groups, Group A and Group B, according to the mean alveolar bone height of the patients. Then, the group was subdivided into Group 1 and Group 2 according to the mean alveolar bone height of the regions of interest where tissues were collected during the surgical procedure. The characteristics of each group are described in Tables 1 and 2.
Patients' serum IgG titers against each type of bacteria (P. gingivalis, P. intermedia, F. nucleatum, S. mutans, and A. actinomycetemcomitans) were determined by use of ELISA to seek the correlation between periodontal disease severity and serum IgG titer by statistical analysis. For all the bacteria tested, IgG antibody titers were higher in Group B than in Group A. However, there was no significant difference observed between the two groups (Table 3).
The RT-PCR patterns of helper T-cell cytokines (IL-10, IL-4, and IFN-γ) expressed by gingival mononuclear cells are depicted (Fig. 1). There were various expression patterns of cytokines from gingival mononuclear cells among patients. In Group 1, IL-10 was expressed in 4 patients, while in the case of Group 2, IL-10 was expressed in 1 patient, thus demonstrating a significant correlation between the expression of IL-10 and alveolar bone level (P<0.05) (Table 4). IFN-γ expression tended to be more frequently observed in Group 2 than in Group 1. However, significant correlation could not be observed (P>0.05) (Table 5). IL-4 was expressed in 1 patient in Group 1, but again there was no statistical significance (P> 0.05) (Table 6).
The dot immunoblot patterns of serum response to P. gingivalis HSP60 #19 peptide and human HSP60 #19 peptide were evaluated (Fig. 2). Five out of 10 patients in Group A demonstrated serum cross-reactivity to peptide #19 of both P. gingivalis HSP60 and human HSP60 #19 peptide, while 1 out of 16 patients in Group B demonstrated serum cross-reactivity. These patterns of serum cross-reactivity to peptide #19 were significantly correlated to alveolar bone level (P<0.05) (Table 7).
Periodontal disease is initiated and exacerbated by fine tuning of the host inflammatory and the immunological response to the multiple pathogenic bacteria infecting the subgingival niche. However, there has not been a clear explanation of the role of the serum antibody to bacteria in periodontal disease, the correlation between alveolar bone destruction and helper T-cell cytokine, and the role of HSP in the etiology of periodontal disease.
It has been reported in previous studies that the serum IgG titer is higher in patients with chronic or aggressive periodontal disease than in healthy controls [3,4,32]. However, it is not clear if the serum IgG titer correctly reflects disease activity or severity. In the present study, serum antibody titers against 5 key periodontopathogenic bacteria (P. gingivalis, P. intermedia, F. nucleatum, S. mutans, and A. actinomycetemcomitans) and their correlation with alveolar bone height was analyzed. The serum antibody titer was higher in Group B (alveolar bone height was less than 70%) than in Group A (alveolar bone height was greater than 70%) of all the tested organisms, but significant differences could not be delineated. This might be because the comparison has been made within patients manifesting varying degrees of alveolar bone destruction in the present study, while other previous studies have compared patient serum titers to those of the healthy control subjects. It might also be that our observation may imply that the serum antibody titer cannot accurately reflect the current level of alveolar bone as an indicator for periodontal disease severity. Horibe et al. [33] reported that the serum IgG titer against P. gingivalis and P. intermedia was significantly decreased after periodontal treatment. Taubman et al. [9], who performed a longitudinal study, explained that the serum antibody titer against bacteria changed only according to disease activation or periodontal treatment. Anderson et al. [34] observed that, in other disease cases, the increase of the serum antibody titer may defend against pathogenic bacteria, but in the case of periodontal disease, it cannot function as the protector. Based on the results of previous studies, it appeared that the serum antibody titer against specific periodontopathogenic bacteria might increase in proportion to the severity of periodontal disease, but this phenomenon was not be observed in the present study. To clarify the correlation between the serum antibody titer and periodontal disease severity, other criteria for periodontal disease severity may have to be adopted in future study designs.
IFN-γ, one of the type 1 helper T-cell cytokines, promotes the generation of proinflammatory cytokine such as IL-1 and tumor necrosis factor-alpha from gingival mononuclear cells. On the other hand, IL-4 and IL-10, cytokines secreted by type 2 helper T-cells, inhibit pro-inflammatory cytokine and type 1 helper T-cell cytokine [10,35,36]. In recent studies relevant to the role of type 2 helper T-cell cytokine in infection-related inflammatory bone resorption, IL-10 is reported to play a principal role in inhibiting bone resorption, while IL-4 does not take any noticeable role [37-39]. Sasaki et al. [39], who compared IL-10 knockout mice with IL-4 knockout mice for the destructive patterns of alveolar bones in experimentally-induced periodontitis, reported that alveolar bone destruction was remarkable in IL-10 knockout mice, but that there were no significant differences between IL-4 knockout mice and the wild-type control group. Takayanagi et al. [16] reported that IFN-γ disturbed the RANKL-RANK signaling system and rapidly degenerated the functions of RANK adapter protein and tumor necrosis factor receptor associated factor 6, and thus strongly inhibits the differentiation of osteoclasts. However, in recent experiments by humanized nonobese diabetic/severe combined immunodeficiency (NOD/SCID) and diabetic NOD mice systems, IFN-γ stimulated the expression of RANKL leading to an increase in alveolar bone destruction [17-19]. IL-10 was significantly increased in Group 1 (alveolar bone height was greater than 70% in the sampled sites) rather than in Group 2 (alveolar bone height was less than 70%). IL-10 expression was positively correlated with alveolar bone height; however, IL-4 was expressed only in 1 patient of Group 2 on the other hand. Thus there was no correlation between the expression of IL-4 and the alveolar bone height. This finding is consistent with previous studies on type 1 helper T-cell cytokine, though what exact mechanism of IL-4 has not been delineated in the process of alveolar bone destruction. It appears that IL-10 plays a crucial role in modulating the homeostasis of bone metabolism. IFN-γ tended to be expressed more frequently in Group 2 than in Group 1, but a statistically significant correlation could not be observed. This result was not consistent with the results of Teng et al. [17], who reported that alveolar bone destruction and IFN-γ expression showed a positive correlation in periodontal disease. This might be because of a difference in the design of the experimental group: with moderate periodontitis patients in our study and aggressive periodontitis patients in the Teng group [17]. There has not been a clear explanation as to what osteoclast-related RANKL expression has to do with IFN-γ. The exact role of IFN-γ in the progression of periodontal disease has to be clarified further.
As the autoimmune characteristics of periodontal disease have been recently reported, HSP comprises a principal antigen that stimulates the host immunological response. HSP is very well-conserved in sequence, sharing a high degree of sequence homology between bacterial species and all living organisms [20,21]. In particular, HSP60 functions as a principal antigen in various infectious diseases [40]. Early studies on HSP were focused on autoimmune disease caused by immunologic cross-reactivity between bacterial HSP and its human counterpart [24,25]. However, most recently, T-cells responding to cross-reactive epitope of human HSP express the immunoregulatory phenotypes and functions that inhibit the autoimmune responses in the inflammatory disease process [26,27]. Through an experiment in which arthritic Lewis rats were used, the cross-reactive epitope of bacterial HSP60 and human HSP60 exert an important role in the immunoregulatory mechanism of HSP60 [28-30]. Many studies have been carried out with regard to the immunoregulatory mechanism of HSP60 in autoimmune disease including rheumatoid arthritis, atherosclerosis, and diabetes, but there have been only a few studies on the immunoregulatory mechanism of periodontal disease. Recently, Choi et al. [31] have produced a poly-reactive monoclonal antibody to P. gingivalis HSP60, which recognized tan immunodominant epitope (peptide #19) of P. gingivalis HSP60, that cross-reacted with peptide #19 from human HSP60. Thus, we have analyzed patients' sera for cross-reactivity to peptide #19 from both P. gingivalis HSP60 and human HSP60 in Group A and B patients. The sero-reactivity to cross-reactive HSP peptide was more frequent in Group A when compared to Group B. The reactivity of serum to peptide #19 from both P. gingivalis HSP60 and its human counterpart demonstrated a statistically significant positive correlation to alveolar bone height. This result was consistent with those of previous findings in an autoimmune arthritis study. It supports the latest hypothesis that the stimulus of specific cross-reactive epitope (peptide #19 of P. gingivalis HSP60) stimulates the development of regulatory T-cells (expressing CD4+, CD25+, FoxP3+ phenotypes) inducing immune tolerance, and inhibits the alveolar bone destruction. Even though the immunoregulatory mechanism of HSP60 in periodontal disease has not been clarified in detail, it is presumable that T-cells responsive to the cross-reactive epitope peptide may play a pivotal role in regulating the immune responses in the development or prevention of periodontal diseases.
Figures and Tables
Figure 1
Expression pattern of helper T-cell cytokine (interleukin [IL]-10, interferon gamma [IFN-γ], IL-4) by gingival tissues obtained from 25 periodontal patients.

Figure 2
Dot immunoblot patterns of serum to peptide #19 from P. gingivalis heat shock protein (HSP, pg#19) and human HSP60 (hu#19), respectively, in Group A and B patients.
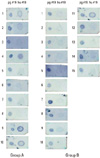
Table 3
Serum IgG antibody titers against tested periodontopathogenic bacteria (enzyme-linked immunosorbent assay unit, mean ± SD).

ACKNOWLEDGMENTS
This work was supported for two years by a Pusan National University Research Grant.
References
2. Ishikawa I, Nakashima K, Koseki T, Nagasawa T, Watanabe H, Arakawa S, et al. Induction of the immune response to periodontopathic bacteria and its role in the pathogenesis of periodontitis. Periodontol 2000. 1997. 14:79–111.

3. Ebersole JL, Taubman MA, Smith DJ, Frey DE. Human immune responses to oral microorganisms: patterns of systemic antibody levels to Bacteroides species. Infect Immun. 1986. 51:507–513.

4. Naito Y, Okuda K, Takazoe I. Immunoglobulin G response to subgingival gram-negative bacteria in human subjects. Infect Immun. 1984. 45:47–51.

5. Ebersole JL, Taubman MA. The protective nature of host responses in periodontal diseases. Periodontol 2000. 1994. 5:112–141.

6. Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL Jr. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998. 25:134–144.

7. Naito Y, Okuda K, Takazoe I, Watanabe H, Ishikawa I. The relationship between serum IgG levels to subgingival gram-negative bacteria and degree of periodontal destruction. J Dent Res. 1985. 64:1306–1310.

8. Kojima T, Yano K, Ishikawa I. Relationship between serum antibody levels and subgingival colonization of Porphyromonas gingivalis in patients with various types of periodontitis. J Periodontol. 1997. 68:618–625.

9. Taubman MA, Haffajee AD, Socransky SS, Smith DJ, Ebersole JL. Longitudinal monitoring of humoral antibody in subjects with destructive periodontal diseases. J Periodontal Res. 1992. 27:511–521.

10. Taubman MA, Kawai T. Involvement of T-lymphocytes in periodontal disease and in direct and indirect induction of bone resorption. Crit Rev Oral Biol Med. 2001. 12:125–135.

11. Kawai T, Matsuyama T, Hosokawa Y, Makihira S, Seki M, Karimbux NY, et al. B and T lymphocytes are the primary sources of RANKL in the bone resorptive lesion of periodontal disease. Am J Pathol. 2006. 169:987–998.

12. Liu D, Yao S, Wise GE. Effect of interleukin-10 on gene expression of osteoclastogenic regulatory molecules in the rat dental follicle. Eur J Oral Sci. 2006. 114:42–49.

13. Al-Rasheed A, Scheerens H, Rennick DM, Fletcher HM, Tatakis DN. Accelerated alveolar bone loss in mice lacking interleukin-10. J Dent Res. 2003. 82:632–635.

14. Al-Rasheed A, Scheerens H, Srivastava AK, Rennick DM, Tatakis DN. Accelerated alveolar bone loss in mice lacking interleukin-10: late onset. J Periodontal Res. 2004. 39:194–198.

15. Sasaki H, Okamatsu Y, Kawai T, Kent R, Taubman M, Stashenko P. The interleukin-10 knockout mouse is highly susceptible to Porphyromonas gingivalis-induced alveolar bone loss. J Periodontal Res. 2004. 39:432–441.

16. Takayanagi H, Ogasawara K, Hida S, Chiba T, Murata S, Sato K, et al. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-gamma. Nature. 2000. 408:600–605.

17. Teng YT, Mahamed D, Singh B. Gamma interferon positively modulates Actinobacillus actinomycetemcomitansspecific RANKL+ CD4+ Th-cell-mediated alveolar bone destruction in vivo. Infect Immun. 2005. 73:3453–3461.

18. Baker PJ, Dixon M, Evans RT, Dufour L, Johnson E, Roopenian DC. CD4(+) T cells and the proinflammatory cytokines gamma interferon and interleukin-6 contribute to alveolar bone loss in mice. Infect Immun. 1999. 67:2804–2809.

19. Mahamed DA, Marleau A, Alnaeeli M, Singh B, Zhang X, Penninger JM, et al. G(-) anaerobes-reactive CD4+ T-cells trigger RANKL-mediated enhanced alveolar bone loss in diabetic NOD mice. Diabetes. 2005. 54:1477–1486.

21. Lamb JR, Bal V, Mendez-Samperio P, Mehlert A, So A, Rothbard J, et al. Stress proteins may provide a link between the immune response to infection and autoimmunity. Int Immunol. 1989. 1:191–196.

22. Choi JI, Chung SW, Kang HS, Rhim BY, Kim SJ. Establishment of Porphyromonas gingivalis heat-shock-protein-specific T-cell lines from atherosclerosis patients. J Dent Res. 2002. 81:344–348.

23. Choi JI, Chung SW, Kang HS, Rhim BY, Park YM, Kim US, et al. Epitope mapping of Porphyromonas gingivalis heat-shock protein and human heat-shock protein in human atherosclerosis. J Dent Res. 2004. 83:936–940.

24. Chung SW, Kang HS, Park HR, Kim SJ, Choi JI. Immune responses to heat shock protein in Porphyromonas gingivalis-infected periodontitis and atherosclerosis patients. J Periodontal Res. 2003. 38:388–393.

25. Ueki K, Tabeta K, Yoshie H, Yamazaki K. Self-heat shock protein 60 induces tumour necrosis factor-alpha in monocyte-derived macrophage: possible role in chronic inflammatory periodontal disease. Clin Exp Immunol. 2002. 127:72–77.

26. Moudgil KD, Durai M. Regulation of autoimmune arthritis by self-heat-shock proteins. Trends Immunol. 2008. 29:412–418.

27. van Eden W, van der Zee R, Prakken B. Heat-shock proteins induce T-cell regulation of chronic inflammation. Nat Rev Immunol. 2005. 5:318–330.

28. Durai M, Gupta RS, Moudgil KD. The T cells specific for the carboxyl-terminal determinants of self (rat) heat-shock protein 65 escape tolerance induction and are involved in regulation of autoimmune arthritis. J Immunol. 2004. 172:2795–2802.

29. Durai M, Kim HR, Bala KK, Moudgil KD. T cells against the pathogenic and protective epitopes of heat-shock protein 65 are crossreactive and display functional similarity: novel aspect of regulation of autoimmune arthritis. J Rheumatol. 2007. 34:2134–2143.
30. Quintana FJ, Carmi P, Mor F, Cohen IR. DNA fragments of the human 60-kDa heat shock protein (HSP60) vaccinate against adjuvant arthritis: identification of a regulatory HSP60 peptide. J Immunol. 2003. 171:3533–3541.

31. Lee JY, Lee JY, Kim SJ, Choi JI. Production and characterization of cross-reactive anti-Porphyromonas gingivalis heat shock protein 60 monoclonal antibody. J Korean Acad Periodontol. 2008. 38:565–578.

32. Ishikawa I, Watanabe H, Horibe M, Izumi Y. Diversity of IgG antibody responses in the patients with various types of periodontitis. Adv Dent Res. 1988. 2:334–338.

33. Horibe M, Watanabe H, Ishikawa I. Effect of periodontal treatments on serum IgG antibody titers against periodontopathic bacteria. J Clin Periodontol. 1995. 22:510–515.

34. Anderson DM, Ebersole JL, Novak MJ. Functional properties of nonhuman primate antibody to Porphyromonas gingivalis. Infect Immun. 1995. 63:3245–3252.

35. Arenzana-Seisdedos F, Virelizier JL, Fiers W. Interferons as macrophage-activating factors. III. Preferential effects of interferon-gamma on the interleukin 1 secretory potential of fresh or aged human monocytes. J Immunol. 1985. 134:2444–2448.
36. te Velde AA, Huijbens RJ, Heije K, de Vries JE, Figdor CG. Interleukin-4 (IL-4) inhibits secretion of IL-1 beta, tumor necrosis factor alpha, and IL-6 by human monocytes. Blood. 1990. 76:1392–1397.

37. Bettelli E, Das MP, Howard ED, Weiner HL, Sobel RA, Kuchroo VK. IL-10 is critical in the regulation of autoimmune encephalomyelitis as demonstrated by studies of IL-10- and IL-4-deficient and transgenic mice. J Immunol. 1998. 161:3299–3306.
38. Kucharzik T, Lugering N, Weigelt H, Adolf M, Domschke W, Stoll R. Immunoregulatory properties of IL-13 in patients with inflammatory bowel disease; comparison with IL-4 and IL-10. Clin Exp Immunol. 1996. 104:483–490.





 PDF
PDF ePub
ePub Citation
Citation Print
Print








 XML Download
XML Download