Abstract
Purpose
The purpose of this study was to observe and quantify the expression of interleukin-4 (IL-4), interferon-γ (IFN-γ), and tissue inhibitor of matrix metalloproteinase-2 (TIMP-2) in the gingival tissue of patients with type 2 diabetes mellitus (DM) and healthy adults with chronic periodontitis.
Methods
Twelve patients with type 2 DM and chronic periodontitis (Group 3), twelve patients with chronic periodontitis (Group 2), and twelve healthy individuals (Group 1) were included in the study. Clinical criteria of gingival (sulcus bleeding index value, probing depths) and radiographic evidences of bone resorption were divided into three groups. The concentrations of cytokines were determined by a western blot analysis and compared using one-way ANOVA followed by Tukey's test.
Chronic periodontal diseases are bacterial infections affecting the periodontium resulting in the loss of tooth support and are associated with bacteremia, inflammation, and a strong immune response. They represent primarily anaerobic Gram-negative oral infection that leads to gingival inflammation, destruction of periodontal tissues and loss of alveolar bone [1,2].
Diabetes mellitus (DM) is a highly prevalent metabolic disorder. The more common form, type 2 diabetes, results from a combination of impaired insulin production and insulin resistance [3].
The mechanisms responsible for these outcomes in patients with diabetes are mainly related to the increased risk of infections, impairment of the synthesis of collagen and glycosaminoglycan by gingival fibroblasts, and increased collagenolytic activity in crevicular fluid [4,5]. Patients with diabetes and periodontitis have enhanced production of inflammatory mediators in the gingival tissues compared to non-diabetics. These changes can contribute to the pathogenesis of periodontal diseases and to alterations in wound healing because collagen is the major structural protein in the periodontium [6,7].
The immune response against periodontopathic bacteria is regulated by the balance between cytokines produced by T helper 1 (Th1) and T helper 2 (Th2) cells. The typical secretory products of Th1 cells are interleukin (IL)-2, IL-12, tumor necrosis factor (TNF)-β, and interferon (IFN)-γ; those of Th2 cells are IL-4, IL-5, IL-6, IL-10, and IL-13 [8].
IL-4 is a glycosylated cytokine secreted by activated T lymphocyte, basophils and mast cells. It is a potent down-regulator of macrophage function [9]. Furthermore IL-4 can down-regulate the CD14 receptor and is also found to induce apoptosis in monocytes. IL-4 also inhibits the IL-1-induced expression of matrix metalloproteinase (MMP)-3 mRNA and protein in human gingival fibroblasts isolated from patients with periodontitis [10].
IFN-γ is an antiviral and antiparasitic agent produced by CD4+/CD8+ lymphocytes and natural killer cells that undergo activation by antigens or mitogens. IFN-γ production modulates T cell growth and differentiation and inhibits the growth of B cells. Synthesis of IFN-γ is inducible by IL-2, fibroblast growth factor, and epidermal growth factor. During the generation of a primary Th1 response, IFN-γ acts as a positive regulator by selectively inducing Th1 differentiation through the increased transcription of T-bet, which results in enhanced IL-12 responsiveness and suppressed Th2 lineage commitment [11]. In some studies [12,13], IFN-γ seemed to be the predominant cytokine produced by T cells in periodontal diseases, and an enhancement of IFN-γ-producing cells was correlated with the progression of disease.
MMPs belong to the matrixin family, which is composed of at least 23 related zinc-dependent endopeptidases that are able to degrade extracellular matrix proteins [14].
Tissue inhibitor of matrix metalloproteinases (TIMPs), which consist of four members, TIMP-1, 2, 3, and 4, have many basic similarities, but they exhibit structural and biochemical differences. These molecules inhibit the proteolytic activity of activated MMPs by forming 1:1 stochiometric inhibitory complex with the enzyme [15]. The balance between activated MMPs and TIMPs controls the extent of extracellular matrix remodeling [16], and a disruption of the MMP-TIMP balance can result in pathological processes such as arthritis, atherosclerosis and periodontitis, in which the loss of extracellular matrix (ECM) is a major feature. TIMP-2 is also able to bind noncovalently to the latent proform of MMP-2 away from its active sites, thereby preventing its activation and inhibiting enzyme activity [17].
Cytokines are considered to play a key role in the inflammation process [18]. In inflammatory response with bone resorption, the role and interactions of IL-4, IFN-γ and TIMP-2 are not clear, and their relative contribution to the pathogenesis of periodontitis and alveolar bone resorption is not entirely established yet. The purpose of this study was to observe and quantify the expression of IL-4, IFN-γ, and TIMP-2 in the gingival tissue of patients with type 2 DM and systemically healthy adults with chronic periodontitis.
The study population consisted of 12 patients with type 2 diabetes and chronic periodontitis (Group 3), 12 patients with chronic periodontitis (Group 2), and 12 healthy individuals (Group 1). Marginal gingival tissue samples were obtained by internal bevel incision at the time of periodontal surgery (including surgical crown lengthening) or tooth extraction and informed consent was obtained from all of the participants before the surgery. This study was approved by the Ethical Committee of Clinical Experiments, Kyungpook National University (74005-1119).
Clinical criteria of gingiva (sulcus bleeding index value, probing depths) and radiographic evidences of bone resorption were divided into three groups according to Joo and Lee's study [19].
Following surgery, excised tissue specimens were immediately placed on liquid nitrogen and subsequently frozen (-70℃).
For western blotting, as previously described by Kim et al. [20] frozen tissues were homogenized in RIPA lysis buffer (10 mM EDTA, 0.15 M NaCl) with 1:30 diluted protease inhibitor cocktail (Roche, Mannheim, Germany) according to Cho et al.'s method [21]. Protein concentrations of supernatant were routinely determined by a Bradford protein assay (Quick Start™, BIO-RAD, Hercules, USA) using bovine serum albumin as standard.
The quantification analysis of IL-4, IFN-γ and TIMP-2 expression was performed using a densitometer (Scion Image β 4.02, Scion Corporation, Frederick, USA). After normalization to β-actin (Abcam, Edinburgh, UK) in each sample, level of IL-4, IFN-γ and TIMP-2 were expressed as a ratio of IL-4, IFN-γ, or TIMP-2/β-actin and the differences of density between the three groups were determined.
All data were presented as means ± SD and results were statistically analyzed. The IL-4, IFN-γ and TIMP-2 levels among the 3 groups were compared using one-way ANOVA followed by Tukey's test. A P-value < 0.05 was considered to be statistically significant.
Both the chronic periodontitis group and the chronic periodontitis with type 2 DM group showed the expression of IL-4, IFN-γ and TIMP-2 in all samples.
IL-4 specific antibodies were used to detect the cytokine in the tissues (Figs. 1A and B). Representative western blot data (Fig. 1A) detected about an 18 kDa molecular weight of IL-4 in all three groups. The expression levels of β-actin were also measured by anti-β-actin specific western blot analysis. In order to quantify the level of IL-4 expression in the groups, the expression levels of IL-4 in each sample were measured by densitometer. Then IL-4 expression levels were normalized by β-actin (ratio of IL-4/β-actin).
The mean amount of IL-4 expression (ratio of IL-4/β-actin) were 0.911 ± 0.131 in Group 1, 0.664 ± 0.155 in Group 2, and 0.648 ± 0.177 in Group 3. There was a significant difference between Groups 1 and 3, and between Groups 2 and 3, but there was no statistically significant difference (P < 0.05) between Groups 1 and 2.
The comparison of IFN-γ expression levels were also made by western blot analysis using IFN-γ specific antibody (Fig. 2A). The levels of IFN-γ expression which detected a molecular weight within the range of 20-25 kDa were also quantified with β-actin normalization (Fig. 2B). The mean amounts of IFN-γ expression (ratio of IFN-γ/β-actin) were 0.465 ± 0.120 in Group 1, 0.553 ± 0.161 in Group 2 and 0.664 ± 0.160 in Group 3. There was a significant difference between Groups 1 and 3 (P < 0.05).
In this study, the molecular weight of TIMP-2 was identified as 21 kDa size in western blot analysis (Fig. 3A). The mean value of TIMP-2 expression (ratio of TIMP-2/β-actin) was 0.407 ± 0.080 for Group 1, 0.559 ± 0.158 for Group 2 and 0.649 ± 0.165 for Group 3. There was a significant difference between Groups 1 and 3, and between Groups 2 and 3 (P < 0.05).
The association between DM and periodontitis has long been discussed with conflicting conclusions. Current studies tend to support a higher incidence and severity of periodontitis in patients with DM. It has been shown that diabetes up-regulates the production of inflammatory cytokines and chemokines [22], leading to increased inflammation, tissue damage, and apoptosis in patients who have periodontitis [23].
The immune response against periodontopathic bacteria is regulated by the balance between cytokines produced by Th1 and Th2 cells. Th1 cells regulate a cell-mediated-type immune response, and Th2 cells regulate a humoral-type immune response. In addition, each subset can regulate the function of the other. The typical secretory products of Th1 cells are IL-2, IL-12, TNF-β, and IFN-γ; those of Th2 cells are IL-4, IL-5, IL-6, IL-10, and IL-13 [8].
IL-4 is a pleiotropic cytokine that inhibits Th1 cells while stimulating a Th2-type of immune response. IL-4 has been shown to inhibit the IL-1 induction of MMP-3 expression in human skin fibroblasts [10]. IL-4 also inhibits the IL-1-induced expression of MMP-3 mRNA and protein in human gingival fibroblast isolated from patients with periodontitis. In a recent study, Salmon-Ehr et al. [24] demonstrated that IL-4, a pleiotropic cytokine, was able to activate connective tissue cells and stimulate accumulation of the extracellular matrix macromolecules. They concluded that IL-4 was implicated in wound healing.
Until now, few studies have reported the expression of IL-4 in the periodontitis patient to be associated with type 2 DM. In this study the quantitative analysis of the IL-4 level showed that IL-4 expression was rather decreased in inflamed gingiva associated with type 2 DM as compared to healthy gingiva and inflamed gingiva of the systemically healthy patient, and the difference was statistically significant (P < 0.05). IL-4 inhibits the secretion of PGE2 and cytokines by macrophage, and suppresses the synthesis of proinflammatory cytokines which induces inflammation, and suggests that the absence of IL-4 induces periodontal disease. This result indicates that IL-4 inhibits inflammatory response in disease progression in chronic periodontitis in type 2 DM patients and plays a role in decreased inflammatory response with bone resorption in patients with this systemic disease.
IFN-γ, released during the early and late stages of the immune response by natural killer cells and activated T cells, respectively, regulates several aspects of the immune response [25]. In addition, it mediates the host defense against infection and is a potent activator of mononuclear phagocytes. In our data, the level of IFN-γ was increased in inflamed gingiva. The amounts of IFN-γ expression were higher in chronic periodontitis patients with type 2 DM as compared to healthy gingiva from a systemically healthy subjects, and the difference was statistically significant (P < 0.05). In some studies, IFN-γ seemed to be the predominant cytokine produced by T cells in periodontal diseases, and an enhancement of IFN-γ-producing cells was correlated with the progression of disease [12]. Gorska et al. [26] reported that the concentration of IFN-γ was significantly higher in serum samples and gingival tissue biopsies from periodontitis patients than from healthy controls. Our data also demonstrated that the total amount of cytokine IFN-γ in active sites in patients with the progression of periodontitis is significantly higher than in inactive sites. IFN-γ is a inflammatory cytokine associated with inflammation, tissue destruction, bone resorption and the production of matrix metalloproteinases and PGE2. The high expression of these cytokines in chronic periodontitis patients may be a marker of continuous Th1 response against bacterial pathogens colonized in gingival tissue, suggesting that the Th1 response plays a destructive role in the periodontium.
The pro-inflammatory cytokines stimulate cells of the host to produce a number of MMPs, which are eventually responsible for degradation of periodontal connective tissues in the pathogenesis of periodontitis. TIMPs, which consist of four members, TIMP-1, 2, 3, and 4, have many basic similarities, but they exhibit structural and biochemical differences. TIMP-2 is able to bind noncovalently to the latent proform of MMP-2 away from its active sites, thereby preventing its activation and inhibiting enzyme activity [17]. An imbalance between MMPs and TIMPs synthesis can promote destruction of the ECM components [16]. The balance between MMP-2 and TIMP-2 expression changes, and several studies have found that during periodontal disease, there is an imbalance of proteinases/inhibitors in favor of proteinases.
Larivee et al. [27] found the concentration of collagenase inhibitors to be higher in healthy gingiva than in periodontitis-affected sites. In this study, the quantitative analysis of TIMP-2 levels showed that TIMP-2 expression was rather increased in inflamed gingiva with or without type 2 DM as compared to healthy gingiva, and the difference was statistically significant. This might suggest that the host is producing TIMP-2 as the anti-proteolytic shield to overcome and regulate the tissue-destructive effects of MMPs in gingival tissues.
In conclusions, this study demonstrated that IFN-γ and TIMP-2 expression levels in human gingival tissue have a positive correlation with the bone resorption process in inflamed tissue and inflamed tissue associated with type 2 DM. This suggests that IFN-γ and TIMP-2 may be involved in the alveolar bone resorptive process of periodontal inflammation associated with type 2 DM. On the other hand, tissue with chronic periodontitis associated with type 2 DM showed significantly decreased IL-4 levels compared to healthy gingiva and non-diabetic inflamed gingiva. This suggests that IL-4 may be involved in the retrogression of periodontal inflammation associated with type 2 DM.
Finally, it seems that more studies are needed to investigate the effect and interrelationship between IL-4, IFN-γ, TIMP-2, and other cytokines that affect the progression of periodontal disease at a higher level. Further studies will contribute to the development of disease diagnosis methods and treatment modalities.
Figures and Tables
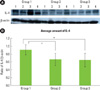 | Figure 1(A) Interleukin (IL)-4 western blot analysis showing 4 representative samples in each group. IL-4 levels were quantified on the basis of β-actin levels. IL-4 corresponding to molecular weight 18 kDa was shown to be expressed in all samples including healthy gingiva, and the expression levels of IL-4 were decreased in order of Groups 1-3. (B) Graphics showing the average amounts (ratio of IL-4/β-actin) and standard deviation of IL-4 level in Groups 1-3. In the healthy gingival tissues (Group 1), the levels of IL-4 were significantly decreased as compared to Groups 1 and 2 (P < 0.05). *Significant difference between Groups 1 and 3 (P < 0.05). †Significant difference between Groups 1 and 2 (P < 0.05). Group 1: healthy gingiva from systemically healthy individuals, Group 2: inflamed gingiva from patients with chronic periodontitis, Group 3: inflamed gingiva from patients with chronic periodontitis and type 2 diabetes mellitus. |
 | Figure 2(A) Interferon (IFN)-γ western blot analysis showing 4 representative samples in each group. IFN-γ levels were quantified on the basis of β-actin levels. IFN-γ corresponding to molecular weight 20-25 kDa was shown to be expressed in all samples including healthy gingiva. The expression levels of IFN-γ increased in order of Groups 1-3. (B) Graphics showing the average amounts (ratio of IFN-γ/β-actin) and standard deviation of IFN-γ level in Groups 1-3. In the inflamed gingiva (with or without diabetes, Groups 2 and 3), the levels of IFN-γ were higher than those in healthy gingiva. *Significant difference between Groups 1 and 3 (P < 0.05). Group 1: healthy gingiva from systemically healthy individuals, Group 2: inflamed gingiva from patients with chronic periodontitis, Group 3: inflamed gingiva from patients with chronic periodontitis and type 2 diabetes mellitus. |
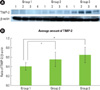 | Figure 3(A) Tissue inhibitor of matrix metalloproteinases-2 (TIMP-2) western blot analysis showing 4 representative samples in each group. TIMP-2 levels were quantified on the basis of β-actin levels. TIMP-2 corresponding to molecular weight 21 kDa was shown to be expressed in all samples including healthy gingiva. The expression levels of TIMP-2 were higher in patients with type 2 diabetes mellitus (DM) than in control healthy subjects. (B) Graphics showing the average amounts (ratio of TIMP-2/β-actin) and standard deviation of TIMP-2 level in Groups 1-3. In the inflamed gingiva (with or without diabetes, Groups 2 and 3), the levels of TIMP-2 were higher than those in healthy gingiva (P < 0.05). *Significant difference between Groups 1 and 3 (P < 0.05). †Significant difference between Groups 1 and 2 (P < 0.05). Group 1: healthy gingiva from systemically healthy individuals, Group 2: inflamed gingiva from patients with chronic periodontitis, Group 3: inflamed gingiva from patients with chronic periodontitis and type 2 DM. |
References
1. Socransky SS, Haffajee AD. The bacterial etiology of destructive periodontal disease: current concepts. J Periodontol. 1992. 63:322–331.

2. Liljenberg B, Lindhe J, Berglundh T, Dahlen G, Jonsson R. Some microbiological, histopathological and immunohistochemical characteristics of progressive periodontal disease. J Clin Periodontol. 1994. 21:720–727.

3. Mealey BL, Ocampo GL. Diabetes mellitus and periodontal disease. Periodontol 2000. 2007. 44:127–153.

4. Diabetes and periodontal diseases. Committee on Research, Science and Therapy. American Academy of Periodontology. J Periodontol. 2000. 71:664–678.
5. Mealey B. Diabetes and periodontal diseases. J Periodontol. 1999. 70:935–949.
6. Nishimura F, Takahashi K, Kurihara M, Takashiba S, Murayama Y. Periodontal disease as a complication of diabetes mellitus. Ann Periodontol. 1998. 3:20–29.

7. Stewart JE, Wager KA, Friedlander AH, Zadeh HH. The effect of periodontal treatment on glycemic control in patients with type 2 diabetes mellitus. J Clin Periodontol. 2001. 28:306–310.

8. Gemmell E, Seymour GJ. Immunoregulatory control of Th1/Th2 cytokine profiles in periodontal disease. Periodontol 2000. 2004. 35:21–41.

9. Shapira L, van Dyke TE, Hart TC. A localized absence of interleukin-4 triggers periodontal disease activity: a novel hypothesis. Med Hypotheses. 1992. 39:319–322.

10. Prontera C, Crescenzi G, Rotilio D. Inhibition by Interleukin-4 of stromelysin expression in human skin fibroblasts: role of PKC. Exp Cell Res. 1996. 224:183–188.

11. Mullen AC, High FA, Hutchins AS, Lee HW, Villarino AV, Livingston DM, et al. Role of T-bet in commitment of TH1 cells before IL-12-dependent selection. Science. 2001. 292:1907–1910.

12. Ukai T, Mori Y, Onoyama M, Hara Y. Immunohistological study of interferon-gamma- and interleukin-4-bearing cells in human periodontitis gingiva. Arch Oral Biol. 2001. 46:901–908.

13. Roberts FA, McCaffery KA, Michalek SM. Profile of cytokine mRNA expression in chronic adult periodontitis. J Dent Res. 1997. 76:1833–1839.

14. Nagase H. Activation mechanisms of matrix metalloproteinases. Biol Chem. 1997. 378:151–160.
15. Hammani K, Blakis A, Morsette D, Bowcock AM, Schmutte C, Henriet P, et al. Structure and characterization of the human tissue inhibitor of metalloproteinases-2 gene. J Biol Chem. 1996. 271:25498–25505.

16. Ryan ME, Ramamurthy S, Golub LM. Matrix metalloproteinases and their inhibition in periodontal treatment. Curr Opin Periodontol. 1996. 3:85–96.
17. Goldberg GI, Marmer BL, Grant GA, Eisen AZ, Wilhelm S, He CS. Human 72-kilodalton type IV collagenase forms a complex with a tissue inhibitor of metalloproteases designated TIMP-2. Proc Natl Acad Sci U S A. 1989. 86:8207–8211.

18. Reynolds JJ, Hembry RM, Meikle MC. Connective tissue degradation in health and periodontal disease and the roles of matrix metalloproteinases and their natural inhibitors. Adv Dent Res. 1994. 8:312–319.

19. Joo SD, Lee JM. The comparison of inflammatory mediator expression in gingival tissues from human chronic periodontitis patients with and without type 2 diabetes mellitus. J Korean Acad Periodontol. 2007. 37:Suppl. 353–369.

20. Kim DH, Park EK, Shin HI, Cho JY, Suh JY, Lee JM. Interrelationship of matrix metalloproteinase and TNF-γ in human gingiva with chronic periodontitis associated to type 2 diabetes mellitus. J Korean Acad Periodontol. 2006. 36:409–405.

21. Cho JY, Xing S, Liu X, Buckwalter TL, Hwa L, Sferra TJ, et al. Expression and activity of human Na+/I- symporter in human glioma cells by adenovirus-mediated gene delivery. Gene Ther. 2000. 7:740–749.

22. Park JW, Lee JM. The comparison of IL-6, elastase and α1-PI expressions in human chronic periodontitis with type 2 diabetes mellitus. J Korean Acad Periodontol. 2007. 37:Suppl. 325–338.

23. Gamonal J, Bascones A, Acevedo A, Blanco E, Silva A. Apoptosis in chronic adult periodontitis analyzed by in situ DNA breaks, electron microscopy, and immunohistochemistry. J Periodontol. 2001. 72:517–525.

24. Salmon-Ehr V, Ramont L, Godeau G, Birembaut P, Guenounou M, Bernard P, et al. Implication of interleukin-4 in wound healing. Lab Invest. 2000. 80:1337–1343.

25. Boehm U, Klamp T, Groot M, Howard JC. Cellular responsesto interferon-gamma. Annu Rev Immunol. 1997. 15:749–795.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download