Abstract
Purpose
Many techniques have been described for achieving vertical augmentation of the maxillary sinus. The aim of this study is to evaluate the effect of low-intensity pulsed ultrasound (LIPUS) to enhance bone regeneration after sinus floor elevation.
Methods
The sinus lifting technique was performed through a lateral approach on 8 different sites of 5 patients (3 males and 2 females) and their mean age was 45.7 years old. The sites were randomly assigned to the control or test groups. The control group had 4 sites that received lateral sinus lifting procedure only, while the test group had 4 sites that received LIPUS application after the lateral sinus lifting procedure. 24-32 weeks (an average of 29 weeks) postoperatively, new bone formation in the augmented sinus sites was evaluated through histologic and histomorphometric analyses of the biopsy specimens obtained during implant placement.
Results
In the test group, the mean percentage of newly formed bone was 19.0±2.8%. In the control group, the mean percentage of newly formed bone was 15.2±3.1%. The percentage of newly formed bone was approximately 4% higher in those cases where the sinus was treated by LIPUS than the percentage in those cases where it was not used. The difference was statistically significant.
Insufficient bone volume is a common problem encountered in the rehabilitation of the edentulous posterior maxillae with implant-supported prostheses [1,2]. These problems have been solved by various surgical methods performed on the maxillary sinus [3-9]. These sinus lift procedures increase bone volume by augmenting the sinus cavity with autogenous bone and/or commercially available biomaterials; many clinicians have tried to use combined materials to maximize the advantages of each material [10-16].
Today, the use of growth factors in conjunction with grafting materials has become the focus of research. Marx [17] introduced a platelet rich plasma (PRP) method, which promotesossification and mineralization in the grafted bone, increases the density of the bone trabecula by 15-30%, releases more growth factors. Also, during the sinus lifting technique, if the PRP is used, it is easier to control the graft material and securely place it, new bone forms more quickly, as does the growth of blood vessels, the recovery of soft tissues quickens, and the PRP itself acts as a biological seal when perforation of the Schneiderian membrane occurs [18-20].
Recently, a procedure was introduced as a method for accelerating the formation of new bone, namely low-intensity pulsed ultrasound (LIPUS), and it is being used in dental surgeries [21-23]. LIPUS is a special type of acoustic pulsed energy that is increasingly used as a supplementary therapy to promote bone and wound healing. LIPUS transmitting as an acoustic pressure wave and applying mechanical stress indirectly to the tissues, has been reported to promote osteogenesis and protein synthesis, calcium uptake, and DNA synthesis in different cells [24]. With these unique characteristics, LIPUS could be applied in many dental surgeries to help promote the formation of new bone.
The aim of this study is to evaluate the efficacy of LIPUS in enhancing bone regeneration in sinus floor elevation.
The product that was used in this experiment is the LIPUS device (BR-Sonic, ITO Co., Tokyo, Japan), which generates LIPUS.
The allograft called Tutoplast (Tutoplast Spongiosa, Tutogen Medical GmbH, Neunkirchen, Germany) and bovine bone substitutes (OCS-B, NIBEC, Seoul, Korea), which have particle sizes of 215-425 µm, were used in the experiment.
The absorbable barrier membrane called Puros Pericardium (Tutoplast, Tutogen Medical GmbH, Neunkirchen, Germany) was chosen for this project. It retains the natural collagen matrix and mechanical properties of natural pericardium.
Before the operation, 10 mL of blood from the patient's vein was extracted and was mixed with 1.5 mL of anticoagulant citrate dextrose (Green Cross, Seoul, Korea) to prevent coagulation. The extracted blood was first centrifuged using the centrifugal separator (Placon, OCT Inc., Seoul, Korea) at 2,000 g for 3 minutes. After the blood was divided into the upper and the lower half of red blood cells, the upper half portion was extracted using a Gilson pipette and centrifuged at 5,000 g for 5 minutes. When this was done, three layers were obtained: the platelet-diluted plasma at the top, the platelet rich buffy coat in the middle, and the leftover blood cells at the bottom. Again, using the Gilson pipette, the top layer containing platelet-diluted plasma was removed. The rest were used to produce 1 mL of PRP including the buffy coat.
The patients included 2 women and 3 men, and their mean age was 45.7 years old. The preoperative diagnosis was made by carrying out panoramic radiography, frequently combined with a CT scan, and the patients were included in the study if no systemic or local contraindications were encountered. Inclusion criteria were a maxillary partial edentulism involving the premolar/molar areas and the presence of a Misch type 3 or 4 sinus situation. Exclusion criteria were acute myocardial infarction within the past 12 months, uncontrolled coagulation disorders, uncontrolled metabolic diseases, radiotherapy to the head within the past 24 months, treatment with intravenous bisphosphonates or with oral bisphosphonates for >3 years, psychiatric problems, heavy smoking (>10 cigarettes/day), alcohol or drug abuse, maxillary sinus pathologies, oral infections, and uncontrolled periodontal disease.
All patients had less than 5 mm of residual alveolar bone height in the posterior maxillary alveolus, and they had also been previously scheduled for the delayed-implant method.
Sinus floor augmentation was carried out on both sides in 3 patients and on one side in 2 cases. Thus, a total of 5 patients with severely atrophic maxillae undergoing 8 sinus lift augmentation procedures were evaluated prospectively. The sites were randomly assigned to the control or test group.
The control group had 4 different areas that received the PRP, barrier membrane, and deproteinized bovine bone and mineral containing Allograft mixed in a ratio of 1:1, and the experimental group had 4 different areas that received the same materials as the control group followed by LIPUS treatment. This protocol was approved by the Institutional Review Board at Dankook University Dental Hospital (H-0706/001/001).
The sinus lifting technique was performed in an altered form of the Caldwell-Luc surgery. Once the patient was under infiltration anesthesia with 2% lidocaine (Huons, Seoul, Korea) containing 1:100,000 of epinephrine, the skin was incised crestally with a #15 scalpel from the maxillary tuberosity area to the mesial tooth area, and then a vertical incision was made to the buccal side followed by a flap reflection, and the bone of the lateral wall of the maxillary sinus in an oval shape was removed with a diamond round bur. Using a maxillary sinus elevation instrument, it was elevated carefully such that the Schneiderian membrane was not perforated. The sinus space was then filled with the mixture of grafting materials and PRP followed by placing the membrane over the side wall of the sinus. The flap was then sutured back with 5-0 ethilon (Ethicon, Summerville, NJ, USA). After the operation, common antibiotics were administered to the patients, and alcohol drinking and smoking were banned; other guidelines were provided including keeping the mouth hygienic at all times. Suture removal was performed 10 days after surgery. During the follow-up treatment, the patient was observed monthly carefully for signs of infection of the implant materials or maxillary sinusitis.
After removing the sutures, the ultrasound therapy was started on the experimental group. The surgical gingiva area was painted with a healing gel and ultrasound was applied to the areas under an audio frequency of 3 MHz at a generation capacity of 240 mW by the BR-Sonic (ITO Co., Tokyo, Japan) device. The LIPUS therapy was repeated every other day for 2 weeks, a total of 7 times, each time lasting for 15 minutes.
After 24-32 weeks (an average of 29 weeks) of healing, the patient was put under local anesthesia with 2% lidocaine containing 1:100,000 of epinephrine and the full thickness flap was elevated to implant the fixture. For all cases in this study, a biopsy of the regenerated tissues was obtained using a standardized internal diameter of 3.75 mm trephine (3i, West Palm Beach, FL, USA) under a cold sterile saline irrigation, starting from the alveolar crest and ending at the most superior part of the graft, at a mean depth of 12 mm, in order to observe the bone regeneration process. Subsequently, screw-type root-form implants were placed into the biopsy osteotomy sites. The collected histologic section was stored in 10% buffered formalin. After the implantation, the flap was replaced and sealed with 5-0 ethilon.
The histological specimens were created as follows: after being fixed in 10% buffered formalin for 10 days, they were washed out with water, then maintained in 5% formic acid for 14 successive days and finally embedded according to the standard technique. The histologic sections were grounded and polished to a final thickness of 40±10 µm, then observed under a microscope after being stained with hematoxylineosin.
Quantitative analysis of bone and bone substitute was performed histomorphometrically using a light microscope. Acquired images were analyzed with an image analyzer (Image Processing Tool Kit, Reindeer Graphics Co., Gainesville, FL, USA). The following measurements were taken: area of bone (area of newly formed bone in proportion to the total measured area); area of grafted materials (area of remaining grafted materials in proportion to the total measured area).
Statistics program (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses. The Wilcoxon signed rank test was used for the comparison of the average new bone volume and grafted material volume for each group. A value of P<0.05 was considered statistically significant.
None of the patients required any additional treatment since they were generally healthy, as they showed no symptoms of infection. In this experiment there was no exposure of the barrier membrane which was used as a cover for the bony window, nor was there leaking of particles from the implant material. There were no hints of perforation or nose bleeding; therefore, it was assumed that there were no perforations in the sinus membrane.
In the control group, grafted deproteinized bovine bone was in the stage of absorption, and the leftover allograft was almost fully attached to the new bone; therefore, it was difficult to discriminate between the allograft and the new bone (Fig. 1).
In the experimental group, the grafted bone was in the stage of absorption, the allograft was resorbed more quickly than deproteinized bovine bone graft. It was easy to witness new bone around both the allograft and xenobone graft, but more was found around the allograft. The shape of the grafted material was not recognizable anymore, and there were no foreign body reactions at all (Fig. 2).
The percentage of newly formed bone in proportion to the total measured area in the control group was 15.2±3.1% and the experimental group was 19.0±2.8% (Table 1).
The difference between the control group and the experimental group was approximately 4%, with the experimental group that experienced LIPUS having the higher rate of bone formation. The difference between the two groups was statistically significant.
The area of the remaining grafted material that had not yet been absorbed in proportion to the total measured area was 21.8±2.0% for the control group and 20.7±3.4% for the experimental group, so the absorption was approximately 1% different (Table 1), but this result was not statistically significant.
Ultrasound treatment raises the temperature in the deep tissues, and is used in the physical therapy field as thermotherapy for pain and muscle spasms. LIPUS is used in pulses for fractures and does not raise the temperature. LIPUS does not use heat to speed up bone formation, but there are reports that the physical pulses mechanically stimulate the cells to be active, causing the cells to absorb as much calcium as they can in order to activate protein kinase A, which may lead to the differentiation of osteoblasts [25-27].
Machen et al. [28] have suggested mechanical, thermal, and electrical generation. Mechanical generation uses micromovements to change the strength of the bone through a passive chemical mediator. Second, for thermal generation, the low intensity ultrasound changes the temperature of the tissues by 1 degree Celsius or less, which changes the activities of enzymes such as collagenase. Third, electrical generation uses low intensity ultrasound to change the electrical potential either through chemical signs or other mechanisms to stimulate bone growth, similar to the investigation of Chapman et al. [29].
The mechanical stimulation of LIPUS leads to the eruption of tissue cells, and when the effects of speeding up bone formation were applied to the sinus lifting technique experiment, the experimental group who received LIPUS showed better results than the controls. The experimental group showed 19.0±2.8% new bone formation, but the control showed only 15.2±3.1%, leaving a 4% higher formation from the experimental group. One of the reasons the experimental group had more bone formation could have been that the LIPUS stimulated more cell activity. Like fibroblasts, chondroblasts, and osteoblasts, a variety of cells respond to a mechanical stimulus and speed up recovery, which fits with the reports that Azuma et al. [30] have presented. Naruse et al. [31] and Wang et al. [32] also reported that LIPUS increases direct anabolism that forms bone matrix. The activities of fibroblasts that are in the periodontal ligament fiber was observed with LIPUS, which stimulated activity, as mentioned in the report of Doan et al. [33]. However, both positive and negative outcomes have been reported for the use of LIPUS, although the ultrasound that is being used in the treatment does not harm the body because it is equal in intensity to the ultrasound image diagnostic system. Overall, LIPUS is safe and offers the benefits of short treatment and fast bone formation.
However, there was a bit of delay to full recovery and there was a limit to the progress of the new bone osseointergration process when comparing both sides of one patient; the above were draw-backs to this experiment. Also, although LIPUS was supposed to be the only experimental factor, differences in technique among surgeons, or differences in remaining bone and sinus form might have affected our results, too. Furthermore, the strength of the ultrasound and its effects might need to be explored more deeply.
In conclusion, according to the limited data of this study, the use of LIPUS seems to be a promising method for enhancing new bone formation for the sinus lifting procedure. However, despite this study demonstrating the enhancing effect of LIPUS on bone regeneration, the biophysical mechanisms involved in the complex bone regeneration process remain unclear and require further research.
Figures and Tables
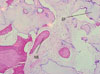 | Figure 1Histologic view of control group (H&E, ×100). Grafted materials (GF) were in the absorption stage, and new bone (NB) around the GF can be seen. |
ACKNOWLEDGMENTS
The present research was conducted with research funding from Dankook University in 2008.
References
1. Bays R. Pathophysiology and anatomy of edentulous bone loss. In : Fonseca RJ, Davis WH, editors. Reconstructive preprosthetic oral and maxillofacial surgery. Philadelphia: Saunders;1986. p. 1–17.
2. Noack N, Willer J, Hoffmann J. Long-term results after placement of dental implants: longitudinal study of 1,964 implants over 16 years. Int J Oral Maxillofac Implants. 1999; 14:748–755.
3. Smiler DG, Johnson PW, Lozada JL, Misch C, Rosenlicht JL, Tatum OH Jr, et al. Sinus lift grafts and endosseous implants: treatment of the atrophic posterior maxilla. Dent Clin North Am. 1992; 36:151–186.
4. Khoury F. Augmentation of the sinus floor with mandibular bone block and simultaneous implantation: a 6-year clinical investigation. Int J Oral Maxillofac Implants. 1999; 14:557–564.
5. Misch CE. Density of bone: effect on treatment plans, surgical approach, healing, and progressive boen loading. Int J Oral Implantol. 1990; 6:23–31.
6. Sennerby L, Thomsen P, Ericson LE. A morphometric and biomechanic comparison of titanium implants inserted in rabbit cortical and cancellous bone. Int J Oral Maxillofac Implants. 1992; 7:62–71.
7. Jemt T, Lekholm U. Oral implant treatment in posterior partially edentulous jaws: a 5-year follow-up report. Int J Oral Maxillofac Implants. 1993; 8:635–640.
8. Boyne PJ, James RA. Grafting of the maxillary sinus floor with autogenous marrow and bone. J Oral Surg. 1980; 38:613–616.
9. Tatum H Jr. Maxillary and sinus implant reconstructions. Dent Clin North Am. 1986; 30:207–229.
10. Wittbjer J, Palmer B, Rohlin M, Thorngren KG. Osteogenetic activity in composite grafts of demineralized compact bone and marrow. Clin Orthop Relat Res. 1983; (173):229–238.

11. Lindholm TS, Nilsson OS, Lindholm TC. Extraskeletal and intraskeletal new bone formation induced by demineralized bone matrix combined with bone marrow cells. Clin Orthop Relat Res. 1982; (171):251–255.

12. Sanders JJ, Sepe WW, Bowers GM, Koch RW, Williams JE, Lekas JS, et al. Clinical evaluation of freeze-dried bone allografts in periodontal osseous defects. Part III. Composite freeze-dried bone allografts with and without autogenous bone grafts. J Periodontol. 1983; 54:1–8.

13. Nasr HF, Aichelmann-Reidy ME, Yukna RA. Bone and bone substitutes. Periodontol 2000. 1999; 19:74–86.

14. Rosen PS, Reynolds MA, Bowers GM. The treatment of intrabony defects with bone grafts. Periodontol 2000. 2000; 22:88–103.

15. Hieu PD, Chung JH, Yim SB, Hong KS. A radiographical study on the changes in height of grafting materials after sinus lift: a comparison between two types of xenogenic materials. J Periodontal Implant Sci. 2010; 40:25–32.

16. Hatano N, Shimizu Y, Ooya K. A clinical long-term radiographic evaluation of graft height changes after maxillary sinus floor augmentation with a 2:1 autogenous bone/xenograft mixture and simultaneous placement of dental implants. Clin Oral Implants Res. 2004; 15:339–345.

17. Marx RE. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001; 10:225–228.

18. Gruber R, Varga F, Fischer MB, Watzek G. Platelets stimulate proliferation of bone cells: involvement of platelet-derived growth factor, microparticles and membranes. Clin Oral Implants Res. 2002; 13:529–535.

19. Lozada JL, Caplanis N, Proussaefs P, Willardsen J, Kammeyer G. Platelet-rich plasma application in sinus graft surgery: Part I--Background and processing techniques. J Oral Implantol. 2001; 27:38–42.

20. Boyapati L, Wang HL. The role of platelet-rich plasma in sinus augmentation: a critical review. Implant Dent. 2006; 15:160–170.

21. Takayama T, Suzuki N, Ikeda K, Shimada T, Suzuki A, Maeno M, et al. Low-intensity pulsed ultrasound stimulates osteogenic differentiation in ROS 17/2.8 cells. Life Sci. 2007; 80:965–971.

22. El-Bialy T, El-Shamy I, Graber TM. Repair of orthodontically induced root resorption by ultrasound in humans. Am J Orthod Dentofacial Orthop. 2004; 126:186–193.

23. Ding Y, Li G, Ao J, Zhou L, Ma Q, Liu Y. 99mTechnetium-methylene diphosphonate bone imaging using low-intensity pulsed ultrasound: promotion of bone formation during mandibular distraction osteogenesis in dogs. Br J Oral Maxillofac Surg. 2010; 48:94–99.

24. Smiler DG, Holmes RE. Sinus lift procedure using porous hydroxyapatite: a preliminary clinical report. J Oral Implantol. 1987; 13:239–253.
25. Leung KS, Cheung WH, Zhang C, Lee KM, Lo HK. Low intensity pulsed ultrasound stimulates osteogenic activity of human periosteal cells. Clin Orthop Relat Res. 2004; (418):253–259.

26. Duarte LR. The stimulation of bone growth by ultrasound. Arch Orthop Trauma Surg. 1983; 101:153–159.

27. Dyson M, Brookes M. Stimulation of bone repair by ultrasound. Ultrasound Med Biol. 1983; Suppl 2. 61–66.
28. Machen MS, Tis JE, Inoue N, Meffert RH, Chao EY, McHale KA. The effect of low intensity pulsed ultrasound on regenerate bone in a less-than-rigid biomechanical environment. Biomed Mater Eng. 2002; 12:239–247.
29. Chapman IV, MacNally NA, Tucker S. Ultrasound-induced changes in rates of influx and efflux of potassium ions in rat thymocytes in vitro. Ultrasound Med Biol. 1980; 6:47–58.

30. Azuma Y, Ito M, Harada Y, Takagi H, Ohta T, Jingushi S. Low-intensity pulsed ultrasound accelerates rat femoral fracture healing by acting on the various cellular reactions in the fracture callus. J Bone Miner Res. 2001; 16:671–680.

31. Naruse K, Miyauchi A, Itoman M, Mikuni-Takagaki Y. Distinct anabolic response of osteoblast to low-intensity pulsed ultrasound. J Bone Miner Res. 2003; 18:360–369.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download