Abstract
Purpose
This study was performed to evaluate the periodontal wound healing effect of particulate equine bone mineral on canine alveolar bone defects.
Methods
Twelve adult male beagle dogs were used as study subjects. The mandibular second and fourth premolars were extracted prior to the experimental surgery, and the extraction sites were allowed to heal for 8 weeks. After periodontal probing, two-walled defects were created at the mesial and distal sides of the mandibular third premolars bilaterally, and the defects were filled with equine particulate bone with collagen membrane or bovine particulate bone with collagen membrane, or collagen membrane alone. The defects without any treatment served as negative controls. After probing depth measurement, animals were sacrificed at 10, 16, and 24 post-surgery weeks for micro-computed tomographic and histomorphometric analysis.
Results
The equine particulate bone-inserted group showed significantly decreased values of probing depth and first bone contact compared to the negative control and collagen membrane alone groups at weeks 10, 16, and 24 (P < 0.05). There were no significant differences in the new cementum length, newly-formed bone area, or newly-formed bone volume between equine particulate bone- and bovine particulate bone-inserted groups, both of which showed significantly increased values compared to the negative control and collagen membrane alone groups (P < 0.05).
Conclusions
Equine particulate bone showed significant differences in probing depth, first bone contact, new cementum length, newly formed bone area, and bone volume fraction values when compared to the negative control and collagen membrane alone groups. There were no significant differences between equine and bovine particulate bone substitutes in these parameters; therefore, we can conclude that equine particulate bone is equivalent to bovine bone for periodontal regeneration.
Modern periodontal treatment modalities have evolved from the repair into the regeneration of periodontal tissue. Alveolar bone destruction is common with severe periodontal disease and is a major cause of tooth loss. To promote bone regeneration in alveolar bone defects around teeth that have suffered from periodontal disease, several types of bone graft materials have been introduced.
Autogenous bone grafts have been referred to as the gold standard and have offered the greatest potential for success in regenerative procedures [1]. This ensures osseous regeneration because it can bear osteogenic, osteoinductive, and osteoconductive properties associated with preosteoblastic cells residing in the graft [2,3]. It is, however, unfavorable to patients and surgeons because it requires additional surgical sites and also has several disadvantages including limited availability, patient morbidity, and irregular resorption rate, which deter general application in clinical practice [4-6]. Hence various allogenic bone substitutes have been developed as alternative candidates for osteoblast migration and proliferation. They still, however, have provoked the fear of disease transmission [7]. This possible shortcoming of allogenic grafts has led the development of alternative graft materials including xenografts [4,8,9].
Deproteinized bovine hydroxyapatite has been documented to be more effective than synthetic alloplasts for promoting new bone formation [10]. Hence, bovine bone mineral has been extensively studied and is widely used in clinics [11,12]. Despite the deproteinization process of bovine bone substitute for preventing possible disease transmission, debate continues over the outbreak of bovine spongiform encephalopathy [13]. Therefore, a need is felt for an alternative type of donor that does not have this risk.
Considering the safety of xenogenic material, an equine-derived bone is proposed to be an alternative xenogenic bone substitute material, which is seldom mentioned in the literature. The purpose of this study was to evaluate the bone regenerative capacity of equine bone mineral on canine alveolar bony defects.
Twelve adult male beagle dogs, weighing around 10 kg each, were used as study subjects. The animals had intact dentition with a healthy periodontium. Animal selection and management, surgical protocol, and preparation followed guidelines approved by the Institutional Animal Care and Use Committee of Seoul National University.
All surgical procedures were performed under general and local anesthesia in sterile conditions with 2% xylazine hydrochloride (Rumpen, Bayer, Seoul, Korea)/ketamine hydrochloride (Ketalar, Yuhan, Seoul, Korea), and 2% lidocaine hydrochloride/epinephrine (1:100,000), respectively. The mandibular second and forth premolars were extracted prior to the experimental surgery and the extraction sites were allowed to heal for 8 weeks. Post extraction care included intramuscular administration of cefazoline sodium, at a dosage of 20 mg/kg (Cefazolin, Yuhan, Seoul, Korea). The extraction sites and remaining dentition received daily oral prophylaxis using 0.2% chlorhexidine (Hexamedin, Bukwang Pharm., Seoul, Korea) topical application.
The experimental surgery, which was performed after periodontal probing, included elevation of the buccal and lingual mucoperiosteal flaps to surgically create two-walled intrabony defects at the mesial and distal sides of the mandibular third premolars bilaterally. The defect, of which dimension was 5 × 5 × 5 mm (mesio-distal width × bucco-lingual width × depth), were created using round and fissure burs with sterile saline coolant (Fig. 1). The defects were filled with equine particulate bone (OCS-H, NIBEC, Seoul, Korea) with collagen membrane (Bio-Gide, Geistlich Pharma AG, Wolhusen, Switzerland) or bovine particulate bone (Bio-Oss, Geistlich Pharma AG, Wolhusen, Switzerland) with collagen membrane, or collagen membrane alone which was used to stabilize the grafted site. The defects without any treatment served as negative controls. Then the flaps were replaced in the original position and sutured. For post-surgical care, cefazoline sodium at a dosage of 20 mg/kg per day and daily topical application of 0.2% chlorhexidine (Hexamedin, Bukwang Pharm., Seoul, Korea) were administered for infection control. The sutures were removed 2 weeks after surgery. Experimental animals were euthanized at 10, 16 and 24 post-surgical weeks after measuring the periodontal probing depth.
Mandibular block sections including the defect sites were collected and images were obtained using a micro-CT machine (Skyscan 1172, Skyscan, Kontich, Belgium). The specimens were arranged in a three-dimensional array not exceeding field-of-view dimensions in order to prevent truncation artifacts. The images obtained were in three-dimensions with an isotropic voxel size of 15 × 1 5 × 15 µm. The X-ray generator was operated at an accelerated potential of 85 kV with a beam current of 116 µA. The X-ray source combines with a two-dimension detector operation at 316 ms of a shutter speed. The three-dimension volume viewer and analyzer software (CT-analyzer, Skyscan, Kontich, Belgium) was used for quantification of newly-formed bone volume (%).
All specimens were dehydrated through a series of ethanol solutions of increasing concentrations and embedded in media (Technovit 7200 VLC, Heraeus Kulzer, Wehrheim, Germany).
Coronal sections were sliced to bear a 30 µm thickness with a grinding machine (EXAKT cutting/grinding systems, EXAKT Advanced Technologies GmbH, Norderstedt, Germany). The sections were stained using toluidine blue. Microscopic observation was done under a light microscope (BX50, Olympus Optical, Osaka, Japan).
For the histomorphometric examination, photographs of each slide were taken using a digital camera (DP71, Olympus Optical, Osaka, Japan) and the resulting images were saved on a computer. Computer-assisted histomorphometric measurements of the newly-formed bone area percentage, newly-formed cementum length, and first bone contact, which was the length from the cementoenamel junction (CEJ) of the designated tooth to the very top of the alveolar bone, were obtained using an automated image analysis system (Scope Eye, Techsantech Co., Seoul, Korea)
Means and standard deviations were calculated for all quantitative data. The collected data of each group was compared by Repeated Measures Analysis of Variance using statistical software package (SPSS, SPSS Inc., Chicago, IL, USA). P < 0.05 was considered to be statically significant.
Probing pocket depth was measured at the proximal sites of the mandibular third molar. There were no significant differences among the baseline probing depth of each group. As can be seen in Table 1, the equine particulate bone-inserted group showed significant probing depth reduction compared to the negative control and collagen membrane alone groups (P < 0.05). There was no significant difference between the bovine bone group and equine bone group.
The alveolar bone defects were mainly filled with fibrous tissues with little new bone formation in the negative control and collagen membrane alone groups compared to the bone substitute inserted groups (Fig. 2). The remaining collagen membrane was not detected in these histologic sections, so it had to have been completely resorbed during the healing period. New cementum formation was evident in both of the particulate bone-inserted groups compared to the control and collagen membrane alone groups.
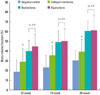
Fig. 3 revealed that the equine particulate bone-inserted groups reached lower first bone contact values than the negative control and collagen membrane alone groups. The equine particulate bone group showed 2.68 ± 0.43 mm, 2.42 ± 0.49 mm, and 0.60 ± 0.36 mm at week 10, 16, and 24, respectively. There was a significant difference from the negative control or collagen membrane alone group at all periods (P < 0.05). There was no significant difference in the first bone contact values between the equine and bovine particulate bone groups. In Fig. 4, the new cementum length was the highest in the equine particulate bone group, with values of 1.95 ± 0.44 mm, 2.88 ± 0.25 mm, and 3.99 ± 0.37 mm at week 10, 16, and 24, respectively. Statistical significance from the negative control existed at all periods (P < 0.05). At week 16 and 24, the equine particulate bone group showed significant new cementum length compared to the collagen membrane alone group (P < 0.05). There is no significant difference in the new cementum length between the equine and bovine particulate bone groups. The newly-formed bone percentage of the equine particulate bone group was prominent, which was 32.05 ± 6.84%, 43.99 ± 9.92%, and 52.99 ± 8.98% at week 10, 16, and 24, respectively (Fig. 5). The collagen membrane alone group showed a significantly increased new bone formation percentage at week 10 and 16, with values of 18.87 ± 8.46% and 25.23 ± 7.23%, respectively, compared to the negative control (P < 0.05). There was no significant difference between the equine and bovine particulate bone groups.
Micro-CT was acquired and analyzed for the bone volume fraction of each group. While the negative control and collagen membrane alone groups showed low values in all observation periods, the equine particulate bone-inserted group showed the highest bone volume fraction, with values at 44.85 ± 12.72%, 50.02 ± 12.53%, and 61.25 ± 15.84% at week 10, 16, and 24, respectively. There was no significant difference between equine and bovine particulate bone groups, both of which showed significant differences compared to the negative control and collagen membrane alone groups (P < 0.05) (Fig. 6).
Among the bone substitute materials, xenografts are widely used because they have an adequate structure relative to the component being replaced and do not compromise the patient's remaining tissues [14,15]. It is previously reported that their physical and chemical properties are similar to those of human bone, so they provide an osteoconductive function [16,17]. Among xenogenic bone substitutes, bovine-derived bone replacement material has been extensively studied and is commonly used in clinical circumstances [11,12,18]. Regarding bovine-derived bone substitutes, however, safety concerns still remain especially with the discovery of bovine spongiform encephalopathy [13]. Considering the safety of xenogenic material, an equine-derived bone is proposed to be an alternative xenogenic bone substitute material, which is rarely reported. One study showed better physical properties of equine block bone than deproteinized bovine block bone [19]. In that study, equine hydroxyapatite and collagen bone blocks were adapted to the shape of the mandibular bone defects of canines. There has been another report that onlay apposition of equine bone blocks appeared to be biocompatible and to be associated with new vessel ingrowth [20]. However, there have been no studies on equine particulate bone substitute as yet. This study is the first report on particulate bone substitutes of equine origin.
Periodontal tissue consists of soft and hard tissue components including gingival epithelium, connective tissues, cementum, and alveolar bone. Periodontal regeneration is hard to achieve because conventional periodontal treatments often result in repair by apical migration of gingival epithelium between the connective tissue and the root surface [21]. In order to regenerate periodontal tissue, the periodontal ligament must attach to the cementum of the tooth while alveolar bone cells proliferate and promote bone formation. For this purpose, we additionally used collagen membranes instead of the bone substitutes alone, the latter of which is not recommended because of the lack of new attachment [22,23]. The possible reason for the appearance of new cementum in the groups with collagen membrane with or without particulate bones can be explained by supplementary use of collagen membrane.
To evaluate the amount of new periodontal attachment, we measured the probing pocket depth just before surgery and before sacrifice. Equine particulate bone, along with bovine particulate bone, showed significantly decreased probing depth compared to the negative control and collagen membrane alone groups. This is an expected result, considering a previous report that the additional use of bone substitutes with membrane resulted in probing depth reduction [24].
The other parameters for periodontal regeneration were examined by quantitative methods in this study. First bone contact, which is the length between the CEJ and the alveolar bone crest, was adopted to confirm whether the probing depth reduction resulted from the alveolar bone formation or connective tissue attachment. In our findings, both particulate bone-inserted groups showed significantly decreased value in this parameter, which confirmed that the probing depth reduction our experiment was mainly due to the alveolar bone regeneration by the bone substitutes. Probing depth reduction might be facilitated by cementum regeneration also, which was evaluated as new cementum length. New cementum length of the equine particulate bone group showed prominent figures, which is another possible explanation for the probing depth reduction in our study.
For quantitative determination of periodontal bone formation, we used both two-dimensional and three-dimensional methods, i.e. measuring newly formed bone area by histomorphometry and bone volume fraction by micro-CT analysis. It has been reported that the structural differences by micro-CT analysis could provide a more accurate assessment in bone regeneration [25-28]. There was consistency in our two-dimensional and three-dimensional results, which showed that equine particulate bone is equivalent to bovine particulate bone in periodontal bone regeneration. Comparing to the control and collagen membrane groups, both bone substitute groups showed significantly increased bone formation values.
In conclusion, both equine bone and bovine bone mineral-inserted groups showed significant differences in probing depth, first bone contact, new cementum length, newly formed bone area, and bone volume fraction values compared to the negative control and collagen membrane alone groups. There were no significant differences between equine bone and bovine bone in those parameters; therefore, we can conclude that equine particulate bone substitute is equivalent to the bovine counterpart within the limits of this study.
Figures and Tables
Figure 1
Two-walled boxtype intrabony defects were surgically created on the mesial and distal side of canine mandibular third premolars (A). After bone mineral was insertion (B), flaps were sutured (C).
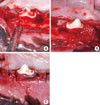
Figure 2
Photomicrographs showing the defects at 10, 16, and 24 weeks. New cementum and bone formation was evident in both particulate bone substitute groups. (A) negative control group, (B) collagen membrane group, (C) bovine particulate bone group, and (D) equine particulate bone group (▴ new bone; ♦ new cementum; ⋆ bone substitutes).
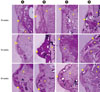
Figure 3
First bone contact (cementoenamel junction to alveolar bone crest) by histomorphometric analysis. Bone mineral-inserted groups showed significantly lower values than the negative control and collagen membrane alone groups. Error bars represent standard deviations.

Figure 4
New cementum length (mm) by histomorphometric analysis. Both of the bone mineral-inserted groups showed significantly higher values compared to the negative control and collagen membrane alone groups. Error bars represent standard deviations.
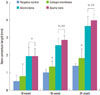
Figure 5
Newly formed bone area by histomorphometric analysis. Both bone mineral-inserted groups showed significantly higher bone formation compared to the negative control and collagen membrane alone groups. Error bars represent standard deviations.
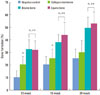
Figure 6
Bone volume fraction (%) of each group. Both bone mineral-inserted groups showed significantly higher bone volume fractions than the negative control and collagen membrane alone groups (P < 0.05). Error bars represent standard deviations.
ACKNOWLEDGEMENTS
The authors thank Mr. Sang-Cheol Lee from Nano-Intelligent Bioengineering Corporation for helpful advice.
References
1. Herold RW, Pashley DH, Cuenin MF, Niagro F, Hokett SD, Peacock ME, et al. The effects of varying degrees of allograft decalcification on cultured porcine osteoclast cells. J Periodontol. 2002; 73:213–219.

2. Dragoo MR, Sullivan HC. A clinical and histological evaluation of autogenous iliac bone grafts in humans. I. Wound healing 2 to 8 months. J Periodontol. 1973; 44:599–613.

3. Hiatt WH, Schallhorn RG. Intraoral transplants of cancellous bone and marrow in periodontal lesions. J Periodontol. 1973; 44:194–208.

4. Valentini P, Abensur D. Maxillary sinus floor elevation for implant placement with demineralized freeze-dried bone and bovine bone (Bio-Oss): a clinical study of 20 patients. Int J Periodontics Restorative Dent. 1997; 17:232–241.
5. Valentini P, Abensur D, Densari D, Graziani JN, Hammerle C. Histological evaluation of Bio-Oss in a 2-stage sinus floor elevation and implantation procedure. A human case report. Clin Oral Implants Res. 1998; 9:59–64.
6. Wang M. Developing bioactive composite materials for tissue replacement. Biomaterials. 2003; 24:2133–2151.

7. Brugnami F, Then PR, Moroi H, Leone CW. Histologic evaluation of human extraction sockets treated with demineralized freeze-dried bone allograft (DFDBA) and cell occlusive membrane. J Periodontol. 1996; 67:821–825.

8. Schwartz Z, Goldstein M, Raviv E, Hirsch A, Ranly DM, Boyan BD. Clinical evaluation of demineralized bone allograft in a hyaluronic acid carrier for sinus lift augmentation in humans: a computed tomography and histomorphometric study. Clin Oral Implants Res. 2007; 18:204–211.

9. Whittaker JM, James RA, Lozada J, Cordova C, GaRey DJ. Histological response and clinical evaluation of heterograft and allograft materials in the elevation of the maxillary sinus for the preparation of endosteal dental implant sites. Simultaneous sinus elevation and root form implantation: an eight-month autopsy report. J Oral Implantol. 1989; 15:141–144.
10. Schmitt JM, Buck DC, Joh SP, Lynch SE, Hollinger JO. Comparison of porous bone mineral and biologically active glass in critical-sized defects. J Periodontol. 1997; 68:1043–1053.

11. Artzi Z, Tal H, Dayan D. Porous bovine bone mineral in healing of human extraction sockets: 2. Histochemical observations at 9 months. J Periodontol. 2001; 72:152–159.

12. Carmagnola D, Adriaens P, Berglundh T. Healing of human extraction sockets filled with Bio-Oss. Clin Oral Implants Res. 2003; 14:137–143.
13. Wenz B, Oesch B, Horst M. Analysis of the risk of transmitting bovine spongiform encephalopathy through bone grafts derived from bovine bone. Biomaterials. 2001; 22:1599–1606.

15. Sogal A, Tofe AJ. Risk assessment of bovine spongiform encephalopathy transmission through bone graft material derived from bovine bone used for dental applications. J Periodontol. 1999; 70:1053–1063.

16. Traini T, Valentini P, Iezzi G, Piattelli A. A histologic and histomorphometric evaluation of anorganic bovine bone retrieved 9 years after a sinus augmentation procedure. J Periodontol. 2007; 78:955–961.

17. Yildirim M, Spiekermann H, Biesterfeld S, Edelhoff D. Maxillary sinus augmentation using xenogenic bone substitute material Bio-Oss in combination with venous blood. A histologic and histomorphometric study in humans. Clin Oral Implants Res. 2000; 11:217–229.

18. Maiorana C, Sigurta D, Mirandola A, Garlini G, Santoro F. Sinus elevation with alloplasts or xenogenic materials and implants: an up-to-4-year clinical and radiologic follow-up. Int J Oral Maxillofac Implants. 2006; 21:426–432.
19. Simion M, Nevins M, Rocchietta I, Fontana F, Maschera E, Schupbach P, et al. Vertical ridge augmentation using an equine block infused with recombinant human platelet-derived growth factor-BB: a histologic study in a canine model. Int J Periodontics Restorative Dent. 2009; 29:245–255.
20. Di Stefano DA, Artese L, Iezzi G, Piattelli A, Pagnutti S, Piccirilli M, et al. Alveolar ridge regeneration with equine spongy bone: a clinical, histological, and immunohistochemical case series. Clin Implant Dent Relat Res. 2009; 11:90–100.

21. Caton J, Nyman S, Zander H. Histometric evaluation of periodontal surgery. II. Connective tissue attachment levels after four regenerative procedures. J Clin Periodontol. 1980; 7:224–231.

22. Dragoo MR, Kaldahl WB. Clinical and histological evaluation of alloplasts and allografts in regenerative periodontal surgery in humans. Int J Periodontics Restorative Dent. 1983; 3:8–29.
23. Froum SJ. Human histologic evaluation of HTR polymer and freeze-dried bone allograft. A case report. J Clin Periodontol. 1996; 23:615–620.

24. Murphy KG, Gunsolley JC. Guided tissue regeneration for the treatment of periodontal intrabony and furcation defects. A systematic review. Ann Periodontol. 2003; 8:266–302.

25. Luo G, Kinney JH, Kaufman JJ, Haupt D, Chiabrera A, Siffert RS. Relationship between plain radiographic patterns and three-dimensional trabecular architecture in the human calcaneus. Osteoporos Int. 1999; 9:339–345.

26. Misch KA, Yi ES, Sarment DP. Accuracy of cone beam computed tomography for periodontal defect measurements. J Periodontol. 2006; 77:1261–1266.

27. Reddy MS. Radiographic alveolar bone change as an outcome measure for therapies that inhibit bone loss or foster bone gain. J Int Acad Periodontol. 2005; 7:175–188.
28. Sant'Anna EF, Gomez DF, Sumner DR, Williams JM, Figueroa AA, Ostric SA, et al. Micro-computed tomography evaluation of the glenoid fossa and mandibular condyle bone after bilateral vertical ramus mandibular distraction in a canine model. J Craniofac Surg. 2006; 17:611–619.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download