Abstract
Purpose
There is no consensus regarding the relationship between the width of keratinized mucosa and the health of peri-implant tissues, but clinicians prefer to provide enough keratinized mucosa around dental implants for long-term implant maintenance. An apically positioned flap during second stage implant surgery is the chosen method of widening the keratinized zone in simple procedures. However, the routine suture techniques used with this method tend to apply tension over the provisional abutments and decrease pre-existing keratinized mucosa. To overcome this shortcoming, a pre-fabricated implant-retained stent was designed to apply vertical pressure on the labial flap and stabilize it in a bucco-apical direction to create a wide keratinized mucous zone.
Methods
During second stage implant surgery, an apically displaced, partial thickness flap with a lingualized incision was retracted. A pre-fabricated stent was clipped over the abutments after connecting to the provisional abutment. Vertical pressure was applied to displace the labial flap. No suture was required and the stent was removed after 10 days.
Results
A clinically relevant amount of keratinized mucosa was achieved around the dental implants. Buccally displaced keratinized mucosa was firmly attached to the underlying periosteum. A slight shrinkage of the keratinized zone was noted after the healing period in one patient, but no discomfort during oral hygiene was reported. Clinically healthy gingiva with enough keratinized mucosa was achieved in both patients.
The significance of keratinized tissue in implant maintenance is a controversial issue, and there is a lack of consensus in the literature regarding the relationship between the width of the keratinized mucosa and the health of peri-implant tissues. Several authors have claimed that there is no correlation between implant success rate and the presence of keratinized tissue in the peri-implant soft tissue [1-3]. On the other hand, some studies have reported that the presence of an adequate band of keratinized tissue adjacent to the implant reduces inflammation [4,5], hyperplasia [5], and retraction of the marginal peri-implant soft tissues [1,5,6]. Despite the fact that a lack of keratinized tissues does not influence the long-term implant survival rate, the presence or reconstruction of keratinized tissue around implants may help facilitate restorative procedures and improve aesthetics [7,8]. In addition, providing enough keratinized tissue around implants enables patients to maintain good oral hygiene without irritation or discomfort during routine oral hygiene [9]. Therefore, clinicians usually seek methods that provide a keratinized mucous zone that is as wide as possible around implants.
Various techniques have been proposed for obtaining adequate amounts of keratinized tissue. Apically or laterally positioned flaps are two techniques focused on preserving existing keratinized tissue [10,11]. When there is not enough keratinized tissue, a free gingival graft (FGG) can be used [10,11]. In shallow vestibules with minimal keratinized tissue, the combination of an apically positioned flap (APF) and FGG can be utilized [12]. However, these techniques have certain limitations, such as a limited quantity of donor tissue [13], frequent infections, patient discomfort, post-surgical pain, paresthesia, bleeding from the donor area, and unpredictable collapse. Most importantly, these methods are highly technique sensitive and time consuming, and cannot be used routinely by general clinicians. To overcome these shortcomings, an easier technique with fewer complications was considered, and a pre-fabricated implant-retained stent (Louis Button®, Dentis, Seoul, Korea) clipped on provisional abutments was developed to easily displace pre-existing keratinized mucosa in the buccoapical direction, preserving the width of the keratinized mucosa and providing flap stabilization without time-consuming and difficult suture techniques. Using this stent, vertical pressure is applied over the displaced buccal flap, reducing the dead space under the flap, helping the displaced gingiva quickly attach to the underlying periosteum, and preserving the width of the pre-existing keratinized mucosa.
The present case report describes a new and simple technique for increasing the width of keratinized mucosa around implants using a pre-fabricated implant-retained stent clipped over provisional abutments.
A 52-year-old woman was referred by the Department of Prosthodontics to the Department of Periodontology, Yonsei University Dental Hospital in April 2008 with a chief complaint of increased mobility on the right second mandibular molar. The patient was in good general health and was a non-smoker. The right second mandibular molar had attachment loss of over 8 mm on the lingual side and degree III mobility. The tooth was diagnosed as a hopeless tooth and was extracted. Healing was left to occur over 3 months and was uneventful. Table 1 shows the patient information and surgical procedures.
Implant surgery was performed by routine procedures in August 2008. After sequential osteotomies, a threaded sandblasted large grit acid-etched (SLA) surface root-form implant (Dentium®, Implantium, Seoul, Korea) was placed (Fig. 1). The implant fixture was submerged and left to heal. The surgery and subsequent healing were uneventful.
Second stage surgery was performed 4 months after the first stage surgery in December 2008. The mobile mucosa was partially covering the area above the implants with a decreased keratinized tissue width (Fig. 2). An APF with a partial thickness flap was chosen for using a pre-fabricated implant-retained stent in order to provide a wider zone of keratinized tissue around the implant. The protocol was approved by the Yonsei Institutional Review Board and informed consent was obtained from the patient after careful explanation of the surgical procedure, prognosis, and possible complications. After the lingualized incision, leaving 4 mm of keratinized mucosa on the buccal flap, a partial thickness flap was reflected. An additional vertical incision was made to maximize the apical displacement of the existing keratinized mucosa (Fig. 3).
The provisional abutment (Ø5.5 × 5 mm) was connected to the fixture, and the pre-fabricated implant-retained stent (Fig. 4) was pushed down over the provisional abutment. A vertical force was applied to stabilize the labial flap and remove the dead space. The lack of stent rotation was confirmed. Originally, the manufacturer recommended that no suture was required. However, simple interrupted sutures (Vicryl® 5.0, Polyglactin 910, Ethicon, Johnson & Johnson, Edinburgh, UK) were made without penetrating the periosteum on the distal side of the implant and over the vertical incision line to maximize stabilization (Fig. 5). However, the displaced flap was mainly stabilized by the stent. The patient was post-operatively administered amoxicillin plus clavulanic acid (Augmentin, 375 mg; Il-Sung Pharm., Seoul, Korea) three times a day for 3 days. The sutures and stent were removed 10 days after surgery, and the patient was recalled for a check-up 1 and 2 months after surgery for post-operative care. At the 1-month check-up, the apically displaced keratinized mucosa appeared firm and stabilized over the underlying tissue. However, a 4-mm-wide band of keratinized mucosa on the buccal side was decreased to 3 mm (Fig. 6). Due to the vertical incision, a step was made on the mesiobuccal side of the implant that happened to show the amount of displacement. At the 2-month check up, the patient was referred to the Department of Prosthodontics for restoration over the implant and increased keratinized mucosa was found to be well maintained at the 6-month check up (Fig. 7).
A 49-year-old woman was referred by the Department of Prosthodontics to the Department of Periodontology in September 2008 with a chief complaint of missing state on #28, #29, and #30. The patient was in good general health and was a non-smoker. An intraoral examination revealed an acceptable oral hygiene status. Initial therapy, including scaling and oral hygiene instruction, was performed.
At the time of the implant surgery, the keratinized mucosa was very narrow and the mucosa was partially covering the buccal side of the edentulous area (Fig. 8). FGG was recommended to the patient, but the patient refused as the procedure could be painful. Therefore, an APF with a partial thickness flap was planned to preserve the pre-existing keratinized mucosa and displace the 3-mm-wide band of keratinized mucosa to the labial side. Informed consent was obtained from the patient. The same surgery protocols were used as in Case 1. Information regarding implant fixture size (Dentium®, Implantium, Seoul, Korea) and the provisional abutment is provided in Table 1. No suture was used in this case and the displaced flap was solely stabilized by the stent. The implant surgery and subsequent healing were uneventful.
The stent was removed after 10 days and there was no sign of infection. The displaced labial flap was already secured over the underlying periosteum. The patient was referred to the Department of Prosthodontics for restoration after 2 months.
Lang and Loe [14] determined how much keratinized gingiva is required to maintain gingival health; inflammation persists in areas with less than 2.0 mm of keratinized gingiva, regardless of the patient's oral health. However, de Trey and Bernimoulin [15] proposed that the width cannot be the only factor deciding the adequacy of the attached gingiva. Other factors, such as the patient's age, oral hygiene capability, aesthetic considerations, and patient's expectations, should be
considered, too [16].
Despite reports that a lack of keratinized mucosa may not influence the long-term survival rate of implants [17,18], the presence and reconstruction of keratinized mucosa around implants seems to reduce the discomfort and irritation of patients during oral hygiene. Thus, clinicians consider it essential to provide enough keratinized mucosa in the long-term maintenance of implants, especially in patients who are not able to maintain adequate oral hygiene [9].
To our knowledge, no specific amount of keratinized mucosa has been recommended as an adequate amount [19]. Clinicians usually choose an appropriate technique to maximize the width of keratinized mucosa from the various available methods, such as an APF, laterally positioned flap, FGG, apically positioned partial thickness flap, or connective tissue graft. However, some of these methods are highly technique sensitive and time-consuming, and the suturing procedures are difficult and complicated. Therefore, APF with a partial thickness flap is preferred in general clinics when a minimum band of existing keratinized mucosa exists. This technique requires no graft or complicated sutures and causes less pain to the patient. The method also reduces the overall operation time and produces acceptable results. However, the periosteal sutures used in this method to stabilize the displaced flap are technique sensitive and time-consuming, and also lack vertical pressure over the displaced flap, creating dead space. This context delays the healing process and sometimes induces necrosis. Therefore, the pre-fabricated implant-retained stent was designed to overcome these shortcomings (Fig. 9) and to be used easily in general clinics without any laboratory equipment, such as other stents used in mucogingival surgeries [20-25].
In these reported cases, the pre-existing narrow band of keratinized mucosa was moved bucco-apically by the APF with a partial thickness flap and stabilized by the stent. The first clinical case had a vertical incision on the mesial side of the implant during the second stage surgery and additional sutures were placed. We decided to make two additional sutures, mesially and distally, to provide additional anchorage. The labial flap, however, was mainly held stable by the stent.
In the second case, the keratinized mucous zone was quite narrow. The remaining band of the keratinized zone was 5 mm wide for #28 and 3 mm wide for #30. The lingualized incision would seem to provide 2-3 mm of keratinized mucosa on the labial flap. After retracting the partial thickness flap, the stents were clipped on the provisional abutment. The labial flap was pushed bucco-apically and the collapsed gingival contour was restored. The increased keratinized mucosa was firmly maintained at a 6-month check up.
Further controlled investigations on a larger patient population are needed to define the efficacy of this stent. Within the limitation of these cases, the pre-fabricated implant-retained stent had the following advantages: 1) The stent displaced the labial flap bucco-apically with the existing keratinized mucous band and secured its position; 2) the flap was flattened over the underlying periosteum and prevented shrinkage and reduction of the keratinized mucosa; 3) the operation time and effort was critically reduced due to no need for periosteal or vertical line sutures; 4) the secondary healing area was partially covered by the stent and food impaction or pain was decreased; and 5) the size of the stent was pre-fabricated and standardized according to the size of the provisional abutments the clinician used.
Even though no complication was noted in these reported cases, the authors have agreed that there might be certain disadvantages to this procedure that require precautions before or during usage: 1) dental implant stability must be obtained to securely attach the stent over the provisional abutment; 2) application in the aesthetic region should be avoided; 3) the stent increases the risk of infection in patients with poor oral hygiene or who smoke, so professional prophylaxis must be provided; and 4) there is a chance the stent may rotate or slip out of the abutment. When these precautions are followed properly, this technique could be potentially used in routine implant procedures to provide enough keratinized mucosa.
Figures and Tables
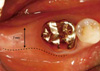 | Figure 2Clinical photo taken 4 months post-implantation. There was a decreased keratinized mucous zone and the buccal side of #31 was partially covered with mucosa. |
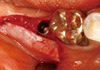 | Figure 3After lingualized incision and retraction of the partial thickness flap, the implant fixture was uncovered. |
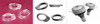 | Figure 4Clinical photograph and three dimensional schematic drawing of the pre-fabricated implant-retained stent. |
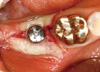 | Figure 5The stent was stabilized over the healing abutment. Additional sutures were placed on the mesial and distal side. |
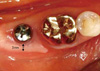 | Figure 6Clinical photo taken 1 month post-surgery. Slight shrinkage of the keratinized mucous zone was noted. |
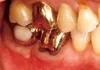 | Figure 7The final restoration was seated 3 months post-surgery. The apically positioned keratinized mucosa was well maintained. |
 | Figure 8Clinical photo of the implant area (A) before first stage implant surgery and (B) 4 months after first stage surgery. (C) Clinical photo of the provisional abutment connection with pre-fabricated implant-retained stent and (D) 2 months after second stage surgery. |
 | Figure 9Comparison of two techniques using an apically positioned flap (APF) with a partial thickness flap around the dental implants. (A) Periosteal and vertical line sutures. Dead space is created under the displaced flap and sutures are difficult and time-consuming. (B) The prefabricated implant-retained stent in the APF with partial thickness flap. Dead space is eliminated by vertical pressure from the stent, and no suture is required on vertical incisions. |
ACKNOWLEDGEMENTS
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (No. R13-2003-013-04002-0).
References
1. Adell R, Lekholm U, Rockler B, Branemark PI, Lindhe J, Eriksson B, et al. Marginal tissue reactions at osseointegrated titanium fixtures (I). A 3-year longitudinal prospective study. Int J Oral Maxillofac Surg. 1986. 15:39–52.
2. Lekholm U, Adell R, Lindhe J, Branemark PI, Eriksson B, Rockler B, et al. Marginal tissue reactions at osseointegrated titanium fixtures. (II) A cross-sectional retrospective study. Int J Oral Maxillofac Surg. 1986. 15:53–61.
3. Schou S, Holmstrup P, Hjorting-Hansen E, Lang NP. Plaque-induced marginal tissue reactions of osseointegrated oral implants: a review of the literature. Clin Oral Implants Res. 1992. 3:149–161.

4. Warrer K, Buser D, Lang NP, Karring T. Plaque-induced peri-implantitis in the presence or absence of keratinized mucosa. An experimental study in monkeys. Clin Oral Implants Res. 1995. 6:131–138.

5. Zarb GA, Schmitt A. The longitudinal clinical effectiveness of osseointegrated dental implants: the Toronto study. Part III: Problems and complications encountered. J Prosthet Dent. 1990. 64:185–194.

6. Adell R, Lekholm U, Rockler B, Branemark PI. A 15-year study of osseointegrated implants in the treatment of the edentulous jaw. Int J Oral Surg. 1981. 10:387–416.

7. Block MS, Kent JN. Factors associated with soft- and hard-tissue compromise of endosseous implants. J Oral Maxillofac Surg. 1990. 48:1153–1160.

8. Buser D, Weber HP, Lang NP. Tissue integration of non-submerged implants. 1-year results of a prospective study with 100 ITI hollow-cylinder and hollow-screw implants. Clin Oral Implants Res. 1990. 1:33–40.

9. Albrektsson T, Zarb G, Worthington P, Eriksson AR. The long-term efficacy of currently used dental implants: a review and proposed criteria of success. Int J Oral Maxillofac Implants. 1986. 1:11–25.
10. Langer B, Sullivan DY. Osseointegration: its impact on the interrelationship of periodontics and restorative dentistry: Part I. Int J Periodontics Restorative Dent. 1989. 9:84–105.
11. Langer B, Langer L. Overlapped flap: a surgical modification for implant fixture installation. Int J Periodontics Restorative Dent. 1990. 10:208–215.
12. Landi L, Sabatucci D. Plastic surgery at the time of membrane removal around mandibular endosseous implants: a modified technique for implant uncovering. Int J Periodontics Restorative Dent. 2001. 21:280–287.
13. Reiser G, Brun J, Mahan P, Larkin L. The subepithelial connective tissue graft palatal donor site:Anatomic considerations for surgeons. Int J Periodontics Restorative Dent. 1996. 16:130–137.
14. Lang NP, Loe H. The relationship between the width of keratinized gingiva and gingival health. J Periodontol. 1972. 43:623–627.

15. de Trey E, Bernimoulin JP. Influence of free gingival grafts on the health of the marginal gingiva. J Clin Periodontol. 1980. 7:381–393.

17. Wennstrom JL, Bengazi F, Lekholm U. The influence of the masticatory mucosa on the peri-implant soft tissue condition. Clin Oral Implants Res. 1994. 5:1–8.

18. Bengazi F, Wennstrom JL, Lekholm U. Recession of the soft tissue margin at oral implants. A 2-year longitudinal prospective study. Clin Oral Implants Res. 1996. 7:303–310.

19. Sclar AG. Soft tissue and esthetic considerations in implant dentistry. 2003. Chicago: Quintessence Publishing Co..
20. Moore JR. A modification of stent design for preprosthetic surgery. J Oral Surg. 1970. 28:263–266.
21. Sanders B, Starshak TJ. Modified technique for palatal mucosal grafts in mandibular labial vestibuloplasty. J Oral Surg. 1975. 33:950–952.
22. Firtell DN, Oatis GW, Curtis TA, Sugg WE Jr. A stent for a split-thickness skin graft vestibuloplasty. J Prosthet Dent. 1976. 36:204–210.

23. Hughes WG, Howard CW 3rd. Simultaneous split-thickness skin grafting and placement of endosteal implants in the edentulous mandible: a preliminary report. J Oral Maxillofac Surg. 1992. 50:448–451.

24. Nystrom E, Kahnberg KE, Albrektsson T. Treatment of the severely resorbed maxillae with bone graft and titanium implants: histologic review of autopsy specimens. Int J Oral Maxillofac Implants. 1993. 8:167–172.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download